Abstract
Background
The incidence of mosquito-borne infections has increased worldwide. Mainland Portugal’s characteristics might favour the (re)emergence of mosquito-borne diseases. This study aimed to characterize the spatial distribution of vectors and notification rates of imported cases of mosquito-borne infections in mainland Portugal and demarcate the areas where these geographies overlap.
Methods
We used data from imported cases of malaria, dengue and Zika from 2009 to 2019, alongside data on the presence of mosquitoes capable of potentially transmitting these diseases at the municipality level (2009–2018). This data was provided by the National Epidemiological Surveillance System and Regional Health Administrations, based on reports from the Vector Surveillance Network. While the mosquitoes in question do not currently transmit these diseases, they have the potential to do so if there is a significant increase in pathogen circulation. A spatial cluster analysis was performed using the univariate Local Moran Index, the Bivariate Moran Local Index and the Mann-Kendall method.
Results
We found significant spatial variability in both notification rates of imported mosquito-borne infections and the distribution of competent mosquito species. We identified clusters of simultaneous high concentrations of vectors and imported cases of malaria in Condeixa-a-Nova (Coimbra), Cuba (Beja), Santiago do Cacém (Setúbal), Albufeira and São Brás de Alportel (Faro), most located on the Southern coast of Portugal. For dengue, we detected clusters of simultaneous high concentrations of vectors and imported cases in Paredes, in the Northern region, and Faro, on the southern coast. For Zika, no clusters were identified.
Conclusion
This study identified areas with high notification rates of imported cases and the presence of competent vectors. Surveillance, control, and awareness efforts are essential, as these areas may present higher risks for local transmission in the future if ecological conditions remain or become suitable, potentially evolving into foci for disease transmission.
Keywords: Vector-Borne diseases, Spatial analysis, Clusters, Spatiotemporal distribution
Background
Diseases transmitted by mosquitoes are a growing public health problem worldwide [1, 2]. The incidence of infectious diseases transmitted by mosquitoes has been increasing due, among other factors, to climate change, urbanization, environmental degradation and increased human mobility [3, 4]. Some of these factors explain the appearance of vectors and diseases transmitted by mosquitoes in new regions, particularly in areas of the northern hemisphere, and the increase in incidence in endemic areas [3, 5].
The interaction between climatic factors, entomological characteristics, and human mobility and behavioural patterns creates a dynamic environment for the transmission and persistence of these diseases [6]. These complex dynamics require a comprehensive examination of the spatiotemporal distribution of cases of infection in humans and the mosquito vectors that transmit diseases, such as malaria, dengue and Zika. In this context, spatial analysis using Geographic Information Systems (GIS) and spatial statistics can be effectively used in surveillance and epidemiological investigation, as it offers great value in studying the spatiotemporal patterns of mosquito-borne infections and the distribution of mosquito vectors [7, 8].
Using spatial analysis to monitor disease and vector distribution dynamics can help predict disease outbreaks and plan public health interventions [9]. Knowledge about the density of spatial clusters and their location helps public health decision-makers understand where to implement site-specific policies and prevention measures to prevent outbreaks from emerging [10, 11]. However, to date, the number of studies that have used spatial analysis methods to investigate diseases transmitted by mosquitoes in Europe is still limited, since researchers focus more on endemic areas and give less importance to new areas where epidemics might emerge [8, 12].
The identification and measurement of areas where there is a significant spatial overlap between high rates of imported cases and regions with high concentrations of mosquito vectors are crucial for improving our capacity to prioritize mosquito control and disease prevention efforts, especially in non-endemic areas [13]. In many cases, these imported infections might lead to localized outbreaks, if ecological suitability is present, emphasizing the need for targeted interventions. Moreover, a better understanding of the spatiotemporal distribution of imported cases will provide critical insights into the epidemiological characteristics of these diseases, which is essential for preventing and controlling outbreaks while ensuring the allocation of sufficient public health resources [14].
In mainland Portugal, so far, 41 species of mosquitoes have been identified. Portugal, being a climate change hotspot [12], may experience increased mosquito density due to rising temperatures and extended breeding periods [15]. This scenario creates favourable conditions for the (re)emergence of diseases transmitted by mosquitoes. It is important to note that malaria was endemic until 1959 and outbreaks of yellow fever were recorded in the 19th century [16, 17]. Moreover, in the last decade, numerous imported cases of Zika, malaria and dengue have been recorded with an increasing trend [18–20].
The introduction of exotic mosquito vector species, such as Aedes albopictus and Aedes aegypti, also raises concerns as they can transmit diseases like chikungunya, yellow fever, and dengue. This poses challenges to public health and requires strict surveillance [21, 22]. The Ae. albopictus, a competent vector for dengue, chikungunya and Zika diseases, was first detected in Europe in Albania in 1979, and from then on, the introduction and distribution of this mosquito expanded rapidly in many countries. This species is currently distributed throughout all regions of the Mediterranean and is expanding to other regions, including northern Europe [23].
Furthermore, the increasing mobility of human populations and the expansion of the tourism sector in Portugal have contributed and will continue to contribute to the importation of cases of mosquito-borne diseases [17, 23–25].
Dengue, chikungunya and Zika are the most imported infections in Europe, due to the large volume of travellers coming and going from endemic areas. Moreover, the spread of invasive mosquito species might lead to the circulation of exotic diseases in Europe [23].
These challenges require continued public health surveillance. However, to date, few articles in the research literature have provided spatiotemporal data on infection cases, as well as on the population size and dynamics of mosquito species relevant to the transmission of mosquito-borne diseases in Portugal [17, 26–29].
Therefore, the objective of this study was to characterize the spatiotemporal distribution of imported cases of mosquito-borne infections and potential mosquito vectors in mainland Portugal and demarcate the areas where these geographies overlap. The resultant maps might serve as practical tools for public health authorities, guiding strategies for mosquito surveillance and allowing for more effective prevention measures by prioritizing areas where the potential for disease transmission might be higher if they are and/or become ecologically suitable. By doing so, this research contributes to ongoing efforts to enhance public health surveillance, preparedness and response, particularly in regions where the (re)emergence of these diseases is becoming increasingly likely due to environmental and climatic changes, such as mainland Portugal.
Methods
Data sources
Reported human cases
To achieve the objective, we used data on imported cases notified in mainland Portugal for the period between 01/01/2009 and 31/12/2019. Mainland Portugal has 278 municipalities and had an average of 9,903,077 inhabitants during the study period, according to Statistics Portugal (Instituto Nacional de Estatística, INE). Only the continental part of Portugal was selected due to its spatial contiguity and the distinct environmental characteristics of the Azores and Madeira archipelagos.
The following infections were considered – malaria, dengue and Zika – chosen because they are the ones that are transmissible by mosquito vectors present in Portugal.
Data was collected and made available by the National Epidemiological Surveillance System (SINAVE). This application system is designed to digitalize the notification process for mandatory reporting of infectious diseases, including those resistant to antimicrobials. It automatically interacts with healthcare-associated infection surveillance systems. SINAVE began its activity in 2014 and its aim is to gather information on communicable diseases and other risks to public health in Portugal [30]. SINAVE works together with doctors, public health services, laboratories, health authorities, and other public, private and social institutions [31]. An electronic platform supports the system by collecting, analysing, interpreting and communicating data on communicable diseases that are mandatory to report in real-time, such as dengue, malaria and Zika. Regional and national disease transmission trends are identified and the need for national prevention and control programs is monitored [32]. National-level results are reported to the World Health Organization (WHO) and the European Centre for Disease Prevention and Control (ECDC), providing disease information to the international community to help prevent and control outbreaks, plan international programs to reduce the impact of communicable diseases on health [33, 34].
In addition to the counts of imported cases according to the municipality, SINAVE also collects data on patients’ sociodemographic, sexual behaviour, geographic, and clinical variables [35]. These variables include sex, age, date of notification, nationality, profession, and the likely origin of the infection (if acquired outside of Portugal).
Notification rates per 1 million inhabitants were calculated for each infection, dividing the total number of cases by the resident population during the corresponding periods, whose estimates are provided by Statistics Portugal [36]. Regarding spatial analyses, we used the municipality of the patient’s residence. We spatially analysed data from 2009 until the end of 2019. To avoid the Small Number Problem, a smoothing method was applied to the obtained notification rates, which improves the precision of the rates by borrowing the strength of other observations. Specifically, an Empirical Bayes approach was applied in combination with a spatial average approach. Spatial weights were determined based on queen contiguity adjacency.
Mosquitoes collected
Data on the number of mosquitoes with the potential for disease transmission were collected between 2009 and 2019. More specifically, counts were obtained from the total number of adult mosquitoes captured and identified in traps during entomological surveillance activities according to municipality. Although some of the identified potential vectors have not been confirmed as transmitters in mainland Portugal, they are considered possible transmitters, even if they are not currently infected, should there be a higher circulation of pathogens.
This data was provided by the National Epidemiological Surveillance System and the Regional Health Administrations, through reports from the Vector Surveillance Network [32]. This network strengthens capacity across regions to increase knowledge about the vector species present, their distribution and abundance, the impact of climate change, explaining their role as vectors and detect invasive species promptly. The Center for the Study of Vectors and Infectious Diseases is integrated into the National Institute of Health Doutor Ricardo Jorge (INSA). The Ricardo Jorge Institute, as the leading authority in epidemiological surveillance, training and dissemination of knowledge, participates in the Vector Surveillance Network (Rede de Vigilância de Vetores – REVIVE) by hosting the Centre for Studies on Vectors and Infectious Diseases (CEVDI) [37]. Regional Health Administrations ensure the availability of equipment for entomological surveillance activities. For adult mosquito collections, CDC light traps and BG Sentinel traps (or Mosquitaire and Vector traps), baited or not with carbon dioxide (or other attractants recommended by suppliers), as well as aspirators, are used. In monitoring invasive mosquitoes, particularly Ae. albopictus, ovitraps with oviposition strips are utilized. In mainland Portugal, the most significant period for mosquito presence occurs from May to October. Most collections are conducted during this period of time. In ports and airports, surveillance is year-round, as well as in areas where invasive species have been identified. The sampling locations and frequency are selected by the Regional Health Administrations, based on proximity to human populations, mosquito presence history, impact on human activities, potential breeding sites, and entry points for exotic/invasive species, considering previous experience under the REVIVE program [32].
Due to the lack of data for the years 2016 and 2019 for the Centro region of Portugal, we excluded these two years from the study analysis.
We categorized mosquito species data according to the types of infection they can transmit, as described in Table 1. It is also important to note that malaria is not caused by a single parasite but by a diverse group of species within the genus Plasmodium [38]. It is crucial to recognize that not all Plasmodium species can be transmitted by the same mosquito vectors [39], which has significant implications for understanding the transmission dynamics of the disease. Previous studies have demonstrated that different Plasmodium species interact with various mosquito vectors, impacting transmission [40].
Table 1.
Grouping of the species of mosquitos according to the infection they can transmit
| Malaria | Zika | Dengue |
|---|---|---|
| Anopheles maculipennis | Aedes.albopictus | Aedes albopictus |
| Anopheles plumbeus | ||
| Anopheles claviger | ||
| Anopheles algeriensis |
Spatial and spatiotemporal analyses
Local spatial association indicators (LISA)
To determine whether the observed patterns of the diseases differ significantly from the expected (at random) patterns, we applied the Anselin Local Moran’s I statistic, which identifies statistically significant hot spots, cold spots, and spatial disparities. The underlying null hypothesis of the Anselin Local Moran’s I statistic is that the spatial pattern of the variable is random, i.e., that there is no spatial autocorrelation, meaning that the values of the variable are spatially independent of each other and that observed cluster or other spatial pattern is due to random factors [41]. This statistic geographically represents local groups with extremely high or low rates, comparing the rate of a municipality with that of adjacent municipalities [42]. Positive or negative statistical values demonstrate a homogeneous grouping of high or low rates, respectively [43]. Local Spatial Association Indicators (LISA) are statistics created by Anselin and colleagues [42], whose purpose is to break down global statistics such as Moran’s I into their local components to identify spatially varying observations and outliers. We determined the Cluster and Outlier Analysis (Anselin Local Moran’s I) for the rates of each studied mosquito-borne infection and for the counts of mosquitos capable of transmitting each of the studied infections.
High-high clusters represent municipalities with high notification rates of imported cases surrounded by others with similarly high rates. Low-low clusters indicate municipalities with low notification rates of imported cases, also surrounded by others with low rates. High-low outliers are municipalities with high notification rates of imported cases surrounded by municipalities with low rates, while low-high outliers are those with low notification rates of imported cases surrounded by municipalities with high rates. The same definitions of clusters and outliers apply to the Bivariate Moran Local Index method.
Bivariate Moran local index (BLISA)
Furthermore, the Bivariate Moran Local Index (BLISA) was used to detect areas with a high prevalence of non-autochthonous cases and, at the same time, a high presence of mosquitoes, corresponding to areas where there will potentially be a greater risk of local transmission in the future if they are and/or become ecologically suitable [44]. High-high clusters correspond to municipalities with high rates of imported cases surrounded by municipalities with high concentrations of mosquitos; low-low clusters correspond to municipalities with low rates of imported cases surrounded by municipalities with a low concentration of mosquitos; high-low outliers correspond to municipalities with high rates of imported cases but are surrounded by municipalities with a low concentration of mosquitos; low-high outliers correspond to municipalities with low rates of notification of imported cases surrounded by municipalities with a high concentration of mosquitos. For this analysis, we focus only on the period for which both data on cases and mosquitos was available – 2009 to 2018, excluding the year 2016.
Mann-Kendall test
To examine spatiotemporal trends, we applied the non-parametric Mann-Kendall test [45, 46]. Data were structured into a cube, with each bin representing event counts over specified time intervals. Emerging Hot Spot Analysis visualized trends, using the Mann-Kendall Trend Test to categorize municipalities into hot and cold spots. Analysis intervals were one year for mosquito vectors and for malaria cases, six months for dengue cases, and two months for Zika cases due to lower case numbers. Spatial relationships were defined using contiguity edges, due to the similarity of neighbouring areas. The Mann-Kendall test was applied independently to each municipality to ensure random, independent variables.
Software
Microsoft Excel 365, ArcGIS Pro 2.9 and GeoDa software were used for data processing and mapping. The ArcGIS software was used to analyse the distribution of the vectors and the notification rates, to estimate Moran’s I spatial autocorrelation and to conduct the spatiotemporal analysis using the Mann-Kendall method. The GeoDa software was used to smooth the rates using the Empirical Bayesian method and to estimate Moran’s bivariate local spatial autocorrelation (BLISA).
Results
Descriptive statistics
During the study period, 1051 cases of malaria were recorded, with 2015, 2016 and 2019 being the years with the most cases – 79 cases of dengue (with more cases in 2019) and 20 of Zika (18 cases in 2016) (Table 2). All cases were imported from people who travelled from an endemic country.
Table 2.
Total number of imported cases infected with malaria, Zika and dengue (2009–2019)
| Malaria | Zika | Dengue | |
|---|---|---|---|
| 2009 | 36 | 0 | 0 |
| 2010 | 46 | 0 | 0 |
| 2011 | 47 | 0 | 0 |
| 2012 | 46 | 0 | 0 |
| 2013 | 99 | 0 | 0 |
| 2014 | 85 | 0 | 0 |
| 2015 | 198 | 0 | 13 |
| 2016 | 193 | 18 | 13 |
| 2017 | 90 | 1 | 11 |
| 2018 | 98 | 1 | 14 |
| 2019 | 113 | 0 | 28 |
| Total | 1051 | 20 | 79 |
At the national level, males were most affected by these infectious diseases in all age groups. The 30 to 59 years old age group gathered almost three-quarters of all cases.
The majority of malaria cases likely originated in Angola with 480 cases, followed by Mozambique, Guinea Bissau, and other countries, primarily those located in Africa. For Zika, most of cases originated from Brazil, followed by Colombia, Cape Verde and Martinique. Cases of dengue were from Brazil, Angola, Cape Verde, India, Indonesia, Thailand, among others.
Concerning vectors, 1,838 mosquitoes were identified that transmit malaria, and 154 were identified that transmit dengue and Zika during the study period (Table 3).
Table 3.
Total number of identified mosquitoes (adults) capable of potentially transmitting malaria, Zika and dengue (2009–2018)
| Malaria | Dengue/Zika | |
|---|---|---|
| 2009 | 0 | 0 |
| 2010 | 148 | 0 |
| 2011 | 123 | 0 |
| 2012 | 241 | 0 |
| 2013 | 167 | 0 |
| 2014 | 144 | 0 |
| 2015 | 204 | 0 |
| 2017 | 281 | 57 |
| 2018 | 530 | 97 |
| Total | 1838 | 154 |
*2016 was excluded from the analysis because data on Centro region was not available
In this study, we identified various species of adult mosquitoes across different municipalities. The most prevalent species was Anopheles maculipennis, with a total of 1,524 specimens recorded across multiple municipalities, indicating its widespread dominance. Anopheles algeriensis was also present, with 124 specimens documented across municipalities, though in lower numbers than An. maculipennis. Anopheles claviger had a total of 135 occurrences, suggesting a stable distribution but less widespread presence. Anopheles plumbeus was recorded in smaller numbers, with only 5 specimens, indicating a sporadic presence across the surveyed municipalities. Lastly, Ae. albopictus showed a notable presence, with 154 specimens observed.
Spatial and spatiotemporal analysis of imported mosquito-borne infection notification rates
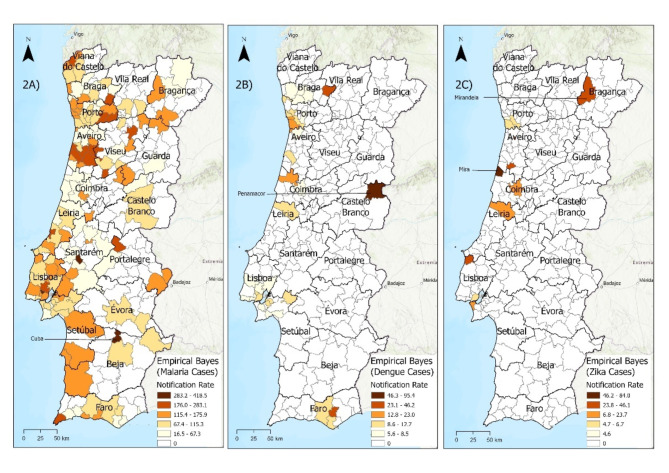
As depicted in Fig. 2A, high notification rates of imported malaria cases are found across Portugal but predominate on the Portuguese coast, mainly in the Vouga basin (Aveiro). The highest notification rate of imported cases was found in Cuba (Beja), with 418 cases per 1,000,000 inhabitants. A depiction of the districts across mainland Portugal is shown in Fig. 1.
Fig. 2.
Notification rates of imported cases smoothed by the empirical Bayes method: 2A) Malaria; 2B) Dengue; 2C) Zika
Fig. 1.
Framework of the districts of mainland Portugal
In the case of dengue (Fig. 2B), the highest notification rates of imported cases were in coastal municipalities, particularly in the districts of Porto and Faro. The highest rate was in Penamacor (Castelo Branco), with 203 cases per 1,000,000 inhabitants.
Regarding Zika (Fig. 2C), the highest notification rates of imported cases of infection are all close to the coast with the sole exception of the region of Mirandela (Bragança), located in the northern interior region. The highest rate was in Mira (Coimbra), with 84 cases per 1,000,000 inhabitants.
Applying the local clustering method (Local I of Moran) to the notification rates of imported cases of malaria (Fig. 3A), we found high-high clusters in the districts of Aveiro, Lisboa and Porto. The low-low clusters were spread across the interior, standing out in the districts of Portalegre, Guarda, Vila Real and the “Pinhal” zone at the junction of the Coimbra-Leiria-Castelo Branco-Santarém districts. In the case of dengue (Fig. 3B), there were two high-high clusters, one at the Porto Metropolitan Area (Matosinhos, Porto, Vila Nova de Gaia and Santa Maria da Feira), the other cluster comprising of Loulé and Faro (Faro). As for Zika (Fig. 3C), low-low classification was detected in Nazaré (Leiria).
Fig. 3.
LISA - Clusters and Outliers of the notification rates of imported cases: 3A) Malaria; 3B) Dengue; 3C)Zika
Mann-Kendall analysis revealed an intensifying hot spot of malaria (Fig. 4A) in the district of Lisbon and in the municipality of Porto. This means that that area was a statistically significant hot spot for 90% of the time-step intervals, including the final time step. Moreover, there were consecutive hot spots in the immediate vicinity. Sporadic hot spots were also observed in these two areas.
Fig. 4.
Mann-Kendall – Total number notifications of imported cases with: 4A) Malaria (2009–2019); 4B) Dengue (2015–2019); 4C) Zika (2016–2019). New Hot Spot (A place identified as a statistically significant hot spot for the first time during the final time step and has never been one before); Consecutive Hot Spot (A location showing a continuous sequence of statistically significant hot spot occurrences in the final time-step intervals. It has not been a statistically significant hot spot before this sequence, and fewer than 90% of all bins are statistically significant hot spots); Intensifying Hot Spot (A location that has been a statistically significant hot spot for 90% of the time-step intervals, including the final time step. In addition, the intensity of clustering of high counts in each time step is increasing overall and that increase is statistically significant); and Sporadic Hot Spot (A location characterized by intermittent hot spot occurrences. Less than 90% of the time-step intervals have been statistically significant hot spots, and none of them has been statistically significant cold spots)
In the case of dengue (Fig. 4B), the municipality of Cascais (Lisboa) was identified as consecutive hot spot occurrences in the final time-step intervals. Sporadic hotspots, by their turn, were located in neighbouring municipalities, and also in the Porto Metropolitan Area. Regarding Zika (Fig. 4C), only sporadic hot spots were found, in the Lisboa and Setúbal districts.
Spatial and spatiotemporal analysis of the mosquito species
Regarding the geographic distribution of adult mosquitoes that can transmit malaria, 1,838 mosquitoes were found in mainland Portugal during the study period, with a greater number in the Sado basin (Setúbal) and Loulé (Faro) (Fig. 5A).
Fig. 5.
Geographic distribution of the total number of mosquito species identified (count) between 2009 and 2018 (2016 as excluded due to incomplete data) capable of potentially transmitting: 5A) Malaria; 5B) Dengue/Zika
With regards to dengue and Zika, 97 adult mosquitoes were identified in the municipality of Loulé and 57 mosquitoes in the municipality of Penafiel (Porto) (Fig. 5B).
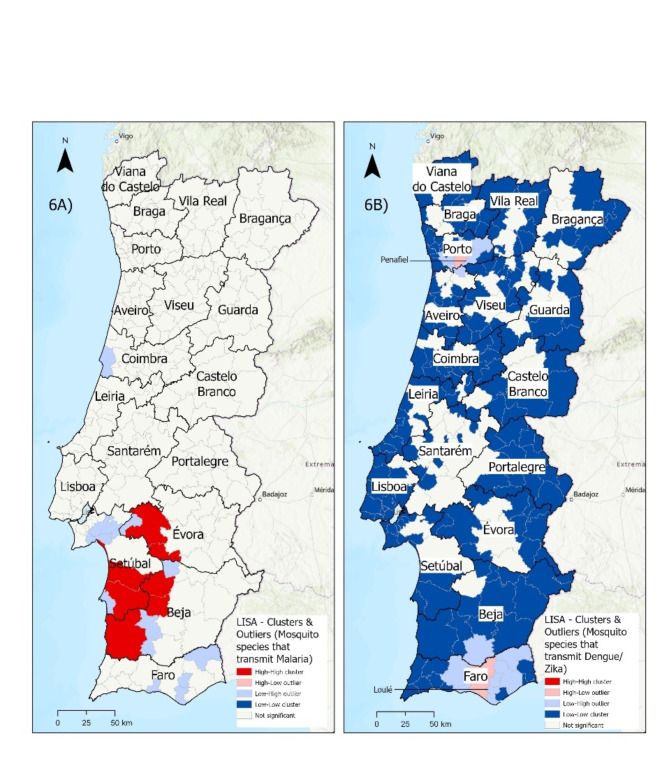
Regarding the malaria vectors, high-high clusters were observed in municipalities situated in the Sado basin and vicinity, belonging to the Setúbal, Évora and Beja districts (Fig. 6A).
Fig. 6.
LISA - Clusters and Outliers of the total number of mosquito species identified (count) between 2009 and 2018 (2016 as excluded due to incomplete data) that transmit: 6A) Malaria; 6B) Dengue/Zika
For the dengue and Zika mosquitoes, no high-high clusters were identified and low-low clusters are spread throughout the country. The municipalities of Loulé (Faro) and Penafiel (Porto) stood out as high-low outliers (Fig. 6B).
When analyzing mosquitos that could transmit malaria (Fig. 7A), consecutive hot spots were identified in the Sado basin and vicinity. Regarding mosquitos known to transmit dengue and Zika (Fig. 7B), new hot spots emerged in the south, namely in the Faro district and consecutive hot spots in the interior of the Porto district.
Fig. 7.
Mann Kendall – Total of mosquito species identified that transmit (2009–2018, 2016 was excluded due to incomplete data): 7A) Malaria; 7B) Dengue/Zika
Bivariate Moran local index to related notifications and mosquito species
The Bivariate Moran Local Index (BLISA) was used to detect areas with a high notification rate of imported cases and, simultaneously, a high presence of mosquitoes.
Concerning malaria, BLISA analysis revealed clusters of simultaneous high concentrations of vectors and high notification rates of imported cases in Condeixa-a-Nova (Coimbra), Cuba (Beja), Santiago do Cacém (Setúbal), Albufeira and São Brás de Alportel (Faro), most located in the southern region of mainland Portugal (Fig. 8A). For dengue, BLISA revealed clusters of simultaneous high concentrations of vectors and high notification rates of imported cases in Paredes, in the North region, and Faro, in the South (Fig. 8B). For Zika, no high clusters were identified (Fig. 8C).
Fig. 8.
Bivariate Moran Local Index (BLISA) of the Notification rates of imported cases and mosquito vectors that transmit (2009 to 2018, 2016 was excluded due to incomplete data): 8A) Malaria; 8B) Dengue; 8C) Zika
Discussion
Our study provides a comprehensive perspective of the spatiotemporal distribution of imported mosquito-borne infectious in mainland Portugal. We found significant spatial variability in the distribution of both notification rates of imported mosquito-borne infections and the distribution of competent mosquito species, as revealed by the spatial and spatiotemporal analyses conducted in this study. Our results and spatiotemporal patterns also varied according to infection and mosquito species.
For malaria, clusters of simultaneous high concentrations of imported cases and vectors were prominent in the Lisbon and Porto Metropolitan Area (intensifying clusters) and Sado basin (consecutive clusters). A total of 1,838 mosquitoes capable of transmitting malaria were identified, with a significant concentration observed in the Sado basin (Setúbal) region. Therefore, according to the Bivariate local index of spatial autocorrelation (BLISA), the Centre and the Southwest of Portugal were identified as areas where local transmission of malaria could potentially emerge in the future if environmental conditions (both biotic and abiotic) are conducive. It is important to note that, in the past, until the second half of the 20th century, autochthonous malaria was present in Portugal, especially in river basins such as Mondego, Tagus, and quite prominently in the Sado basin, associated with the production of rice [47]. A study conducted in Portugal on the malariogenic potential (i.e., the capacity or likelihood of a specific area to support the transmission and resurgence of malaria [17]) revealed that the risk of malaria resurgence exists, although it is concentrated in areas identified as hotspots, more precisely in the Tagus and Sado basins [47, 48]. Similarly, although our studies may not be entirely comparable (due to differences in scope, methods, and data used), we also observed a hotspot in the Sado basin. In 2019, 8,641 cases of malaria were reported in the EU/EEA, and 9 of those cases were reported as acquired in the EU (two each by Germany, Greece, Spain and France, and one by the Netherlands) [49]. Hence, climatic changes, coupled with migratory flows from endemic countries, may increase the risks of malaria (re)emergence in European countries, such as Portugal [8].
Concerning dengue, we identified clusters of high prevalence in the Porto Metropolitan Area (North of Portugal), and in the municipalities of Faro and Loulé, on the southern coast. The mosquitoes that transmit dengue (and also Zika) - Ae. albopictus – were detected in the municipalities of Paredes (located in the North of Portugal) and Faro on the South coast. Accordingly, we observed clusters of simultaneous high concentrations of vectors and high concentrations of imported cases in the municipalities of Paredes (North) and Faro, on the southern coast. The introduction of the mosquito was also detected recently in Alentejo in 2022 and in Lisbon in 2023. Dengue existed in Portugal and Europe until the last century. However, with the eradication of A. aegypti, there were no autochthonous dengue cases until the outbreaks in France and Croatia in 2010, following the introduction of Ae. albopictus [50]. Subsequently, in 2012, the initial dengue outbreak occurred in Madeira, attributed to A. aegypti, where the vector is now established [27]. A review study focusing on Europe indicated that within approximately 30 years, Ae. albopictus will find suitable areas covering 68% of the continent, including the majority of the British Isles, Ireland, and the southern regions of Scandinavian countries. The present suitability of Ae. albopictus has been identified in the northwest of the Iberian Peninsula, southern France, Italy, and the coastline extending from the western Balkans to Greece [19, 51].
Regarding Zika, no significant clusters of high concentrations of imported cases or areas with both high concentrations of imported cases and competent vectors were identified. Only sporadic hotspots were observed in the Lisbon Metropolitan Area. Almost all cases of Zika were imported from Brazil. In Portugal, the risk of Zika importation is elevated due to the historical relations between Portugal and Brazil, characterized by significant trade and travel [52–54].
Spatiotemporal analysis and GIS-generated maps provide relevant information that can help direct monitoring and surveillance efforts and plan public health interventions. Knowledge about the density of spatial clusters and their locations helps public health decision-makers determine where to implement site-specific policies, targeted surveillance and prevention measures to prevent outbreaks from emerging. This study only characterized the distribution of imported cases and mosquitoes and their co-occurrence. Hence, we recognize that our paper is descriptive in nature and has a limited capacity to predict and determine locations with a high risk of local transmission, as our study only accounts for the locations of the competent vectors and imported cases, disregarding other biotic and abiotic factors. Therefore, caution is advised when interpreting these exploratory results. Nevertheless, it constitutes an important first step and helps to delineate hypotheses. Further investigation is now being planned to incorporate those variables in future studies by the team. For instance, international travel and daily mobility are important elements in the study of mosquito-borne disease transmission [55]. Likewise, the environmental or socioeconomic factors that characterise each municipality can also impact the local disease and vector pool [56–58].
Strengths and limitations
Firstly, it is important to recognize that the high concentrations of imported cases in this study may not fully represent the true prevalence of mosquito-borne infections in the population. This limitation arises from several factors, including underreporting due to individuals’ reluctance to seek medical care, challenges in accessing healthcare services, asymptomatic cases, and instances where healthcare is sought outside the country. Furthermore, it is important to recognize that the geographic distribution of notification rates does not represent the risk of local transmission, as these rates are based on imported cases. Instead, this distribution primarily reflects areas with higher travel from endemic regions.
Another limitation of the study was the need to exclude certain periods due to incomplete data. The Regional Administration of Centro was unable to produce the REVIVE reports for 2016 and 2019, forcing us to exclude these two years from the analysis. Another limitation was that the database of reported human cases was provided for each disease over different time periods, as SINAVE was established in 2014, five years after 2009. The only data available between 2009 and the first half of 2014 were for malaria. Consequently, our analysis might be affected by the exclusion of these periods. Additionally, the limited data availability resulted in a reduced number of cases utilized for calculating notification rates in the spatial analysis, potentially influencing the robustness of the findings. Furthermore, the clusters detected in the spatial analysis were explored without taking into consideration other covariates, as we aimed to identify spatial patterns and generate hypotheses regarding underlying risks. As mentioned before, future studies should explore the biotic and abiotic factors that explain the observed patterns. Moreover, the analysis we conducted did not provide species-specific information, as mosquito counts were aggregated and reported by pathogen-specific groups. Consequently, malaria had naturally higher mosquito numbers due to the larger number of species represented in these groups. Additionally, not all mosquito species exhibit the same degree of anthropophilic behaviour. While Ae. albopictus is known for its preference for biting humans, other species detected in the study primarily feed on non-human animals, reducing their potential role in the transmission of malaria to humans. Additionally, it remains unclear whether there is a sampling bias towards certain Portuguese regions, which might partially explain the geographies of the vectors. This bias could stem from the reactive nature of surveillance efforts, often triggered by reports of symptomatic cases in sentinel species that may not be evenly distributed nationwide [29]. Another limitation of our study is that CDC traps are particularly effective for capturing Culex species, while BG traps are optimized for Ae. species. Therefore, other mosquito species may not be adequately represented using these methods. This difference in trap effectiveness may impact the comprehensive accuracy of mosquito surveillance and should be considered in interpreting the data [59, 60].
LISA method is an effective tool for identifying spatial clusters, but it has some limitations in the context of this study. First, it is sensitive to spatial scale and neighbourhood definition, which can influence cluster detection depending on the level of aggregation used. Furthermore, LISA analysis performs multiple statistical tests, increasing the risk of detecting clusters by chance. LISA also does not consider the temporal dimension, which has been an important limitation throughout the study period. Municipalities with little data may result in inconsistent or non-statistically significant results, which limits the robustness of the analysis in areas with a low density of cases. Finally, in very heterogeneous areas, the method may present difficulties in interpreting spatial patterns, especially when different sub-regions have different dynamics. To mitigate these limitations, the application of complementary approaches, such as spatiotemporal analysis or spatial regression, would be recommended [42, 61, 62].
The Mann-Kendall method is useful for detecting trends in time series, especially when working in public health, such as in the case of vector-borne diseases. However, like any statistical method, it has its limitations. Municipalities and regions with fewer cases may show results with little statistical significance due to the small sample size. This is sensitive to short time series and does not identify abrupt changes or provide the magnitude of the trend. The method assumes that the trend is homogeneous across the time series, however, in epidemiological scenarios, trends may vary over time [45, 63, 64].
Despite the limitations, this study can be of great use for public health, as the spatiotemporal identification of areas with a higher risk provides data that enable the adaptation of intervention and prevention programs for cases of infection. It is, to our knowledge, the first study conducted in mainland Portugal that describes the spatiotemporal epidemiology of a wide range of mosquito-borne infections and their vectors.
Although local transmission has not yet occurred for most of the infections studied, our findings can inform several preventative recommendations: (1) strengthen surveillance in critical areas identified by LISA and Mann-Kendall analyses, prioritizing interventions and monitoring in hotspot locations; ((2) conduct further research to develop prediction models based on biotic and abiotic factors; and (3) increase surveillance of at-risk populations, improving awareness among travellers to endemic regions by offering protective measures guidance by health services, and ensuring rapid diagnosis, treatment, and response through malaria screening of travellers [65–69].
Conclusions
In conclusion, this study identified areas with higher notification rates of imported cases of mosquito-borne infections concurrent with the presence of potential vectors, highlighting the importance of continuous monitoring of these regions. However, it is essential to highlight that the identification of these areas does not necessarily imply a risk of a local outbreak, but rather the need for continued surveillance in critical areas identified by LISA and Mann-Kendall analyses, prioritizing interventions and monitoring in hotspot locations and additional studies to better understand the biotic and abiotic factors that may influence local transmission. The findings of this research can greatly assist in public health management and the implementation of intervention strategies, not only in Portugal but also in European regions with similar physical and social characteristics.
Acknowledgements
The authors would like to thank the Directorate-General for Health for providing data from the National Epidemiological Surveillance System (SINAVE) and the Regional Health Administrations of the Algarve, Alentejo, Centro, Lisbon and Vale do Tejo and Norte and the Instituto Ricardo Jorge for providing the Vector Surveillance Network (REVIVE) reports. The authors would like to thank Sarah Murphy for her assistance in reviewing the English language of this manuscript.
Abbreviations
- Ae
Aedes
- An
Anopheles
- BG
Biogents
- BLISA
Bivariate Local Indicators of Spatial Association
- CDC
Centers for Disease Control
- CEVDI
Centro de Estudos de Vetores e Doenças Infeciosas
- ECDC
European Centre for Disease Prevention and Control
- EPIUnit
Unidade de Investigação em Epidemiologia
- EU/EEA
European Union/European Economic Area
- FCT
Fundação para a Ciência e Tecnologia
- GIS
Geographic Information Systems
- INE
Instituto Nacional de Estatística
- ITR
Laboratório para a Investigação Integrativa e Translacional em Saúde Populacional
- LISA
Local Indicators of Spatial Association
- REVIVE
Rede de Vigilância de Vetores
- SINAVE
Sistema Nacional de Vigilância Epidemiológica
- WHO
World Health Organization
Author contributions
AIR conceived and designed the study. SM and AIR performed the analysis and interpreted the data. SM wrote the first draft of the paper. AIR, JR, AG and BG reviewed and approved the final manuscript. All authors have read and approved the final manuscript.
Funding
This work is funded by the Foundation for Science and Technology (FCT) in a Doctoral Scholarship Program under the Geography and Spatial Planning Research Centre. Project ref: 2020.07201.BD. This research received support from the Centre of Studies in Geography and Spatial Planning (CEGOT), funded by national funds through the Foundation for Science and Technology (FCT) under the reference UIDB/04084/2020. Ana Isabel Ribeiro was supported by National Funds through FCT, under the programme of Stimulus of Scientific Employment–Individual Support within the contract CEECIND/02386/2018 (10.54499/CEECIND/02386/2018/CP1538/CT0001). This work was supported by FEDER through the Operational Programme Competitiveness and Internationalisation and national funding from the Foundation for Science and Technology – FCT (Portuguese Ministry of Science, Technology and Higher Education) under the Unidade de Investigação em Epidemiologia - Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (UIDB/04750/2020) and Laboratório para a Investigação Integrativa e Translacional em Saúde Populacional (ITR) (LA/P/0064/2020). Jorge Rocha was financed by National funds through FCT -Portuguese Foundation for Science and Technology, I.P., under the framework of the project “TRIAD - health Risk and social vulnerability to Arboviral Diseases in mainland Portugal” [PTDC/GES -OUT/30210/2017] and by the Research Unit UIDB/00295/2020.
Data availability
The Statistics Portugal datasets on population are available online at Statistics Portugal website (https://www.ine.pt/) The SINAVE and REVIVE datasets can be obtained upon formal request to the respective entities.
Declarations
Ethics approval and consent to participate
Ethical approval was granted by the Public Health Institute of the University of Porto (CE21181). The study also follows the EU General Data Protection Regulation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO: Global Vector Control Response 2017–2030. In., 1. July 2017 edn; 2017.
- 2.WHO. A global brief on vector-borne diseases. In.; 2014.
- 3.INSA. Doenças associadas a artrópodes vetores e roedores. In. Edited by Núncio MS, Alves MJ, 2º edn. Lisboa: Instituto Nacional de Saúde Doutor Ricardo Jorge, IP -Departamento de Doenças Infeciosas - Centro de Estudos de Vetores e Doenças Infeciosas Doutor Francisco Cambournac; 2019.
- 4.Baylis M. Potential impact of climate change on emerging vector-borne and other infections in the UK. Environmental Health 2017, 16(S1).10.1186/s12940-017-0326-1. [DOI] [PMC free article] [PubMed]
- 5.Gyawali N, Bradbury RS, Taylor-Robinson AW. The global spread of Zika virus: is public and media concern justified in regions currently unaffected? Infectious Diseases of Poverty 2016, 5(1).10.1186/s40249-016-0132-y. [DOI] [PMC free article] [PubMed]
- 6.Baker RE, Mahmud AS, Miller IF, Rajeev M, Rasambainarivo F, Rice BL, Takahashi S, Tatem AJ, Wagner CE, Wang L-F et al. Infectious disease in an era of global change. Nature Reviews Microbiology 2022, 20(4):193-205.10.1038/s41579-021-00639-z. [DOI] [PMC free article] [PubMed]
- 7.Molina-Guzmán LP, Gutiérrez-Builes LA, Ríos-Osorio LA. Models of spatial analysis for vector-borne diseases studies: A systematic review. Veterinary World 2022:1975-1989.10.14202/vetworld.2022.1975-1989. [DOI] [PMC free article] [PubMed]
- 8.Moutinho S, Rocha J, Gomes A, Gomes B, Ribeiro AI. Spatial analysis of Mosquito-Borne diseases in Europe: a scoping review. Sustainability. 2022;14(15):8975. [Google Scholar]
- 9.Abdur Rehman N, Salje H, Kraemer MUG, Subramanian L, Saif U, Chunara R. Quantifying the localized relationship between vector containment activities and dengue incidence in a real-world setting: A spatial and time series modelling analysis based on geo-located data from Pakistan. PLoS Negl Trop Dis 2020, 14(5):e0008273.10.1371/journal.pntd.0008273. [DOI] [PMC free article] [PubMed]
- 10.Casas J, Lazzari C, Insausti T, Launois P, Fouque F. Mapping of courses on vector biology and vector-borne diseases systems: time for a worldwide effort. Mem Inst Oswaldo Cruz. 2016;111(11):717–9. 10.1590/0074-02760160295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cromley EK, McLafferty S. GIS and Public Health. Guilford; 2012.
- 12.Tuel A, Eltahir EAB. Why is the Mediterranean a Climate Change Hot Spot? J Clim. 2020;33(14):5829–43. 10.1175/jcli-d-19-0910.1. [Google Scholar]
- 13.Taheri S, González MA, Ruiz-López MJ, Magallanes S, Delacour-Estrella S, Lucientes J, Bueno-Marí R, Martínez-De La Puente J, Bravo-Barriga D, Frontera E et al. Modelling the spatial risk of malaria through probability distribution of < i > Anopheles maculipennis s.l. and imported cases. Emerging Microbes & Infections 2024, 13(1).10.1080/22221751.2024.2343911. [DOI] [PMC free article] [PubMed]
- 14.Lun X, Wang Y, Zhao C, Wu H, Zhu C, Ma D, Xu M, Wang J, Liu Q, Xu L et al. Epidemiological characteristics and temporal-spatial analysis of overseas imported dengue fever cases in outbreak provinces of China, 2005–2019. Infectious Diseases of Poverty 2022, 11(1).10.1186/s40249-022-00937-5. [DOI] [PMC free article] [PubMed]
- 15.Rocha J, Oliveira S, Viana CM, Ribeiro AI. Climate change and its impacts on health, environment and economy. In., edn.: Elsevier; 2022: 253-279.10.1016/b978-0-12-822794-7.00009 – 5.
- 16.Arrow KP, Gelband C. H.: Saving lives, buying Time: Economics of Malaria drugs in an age of resistance. Washington (DC): National Academies Press (US); 2004. [PubMed] [Google Scholar]
- 17.Gomes E, Capinha C, Rocha J, Sousa C. Mapping risk of Malaria Transmission in Mainland Portugal using a Mathematical Modelling Approach. PLoS ONE. 2016;11(11). 10.1371/journal.pone.0164788. [DOI] [PMC free article] [PubMed]
- 18.Paixão ES, Teixeira MG, Rodrigues LC. Zika, Chikungunya and dengue: the causes and threats of new and re-emerging arboviral diseases. BMJ Global Health. 2018;3(Suppl 1):e000530. 10.1136/bmjgh-2017-000530. [DOI] [PMC free article] [PubMed]
- 19.Oliveira S, Rocha J, Sousa CA, Capinha C. Wide and increasing suitability for Aedes albopictus in Europe is congruent across distribution models. Scientific Reports 2021, 11(1).10.1038/s41598-021-89096-5. [DOI] [PMC free article] [PubMed]
- 20.Serrano D, Santos-Reis A, Silva C, Dias A, Dias B, Toscano C, Conceição C, Baptista-Fernandes T, Nogueira F. Imported Malaria in Portugal: prevalence of polymorphisms in the anti-malarial drug resistance genes pfmdr1 and pfk13. Microorganisms. 2021;9(10):2045. 10.3390/microorganisms9102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almeida APGd. Os mosquitos (Diptera, Culicidae) e a sua importância médica em Portugal: desafios para o século XXI. Acta Med Port. 2011;24(6):961–74. [PubMed] [Google Scholar]
- 22.Schaffner F. A Mathis 2014 Dengue and dengue vectors in the WHO European region: past, present, and scenarios for the future. Lancet Infect Dis 14 12 1271–80 10.1016/s1473-3099(14)70834-5. [DOI] [PubMed] [Google Scholar]
- 23.Brugueras S, Fernández-Martínez B, Martínez-De La Puente J, Figuerola J, Porro TM, Rius C, Larrauri A, Gómez-Barroso D. Environmental drivers, climate change and emergent diseases transmitted by mosquitoes and their vectors in southern Europe: A systematic review. Environmental Research 2020, 191:110038.10.1016/j.envres.2020.110038. [DOI] [PubMed]
- 24.Conceição C, Medeiros M, Pereira N, Gonçalves L, Antunes A, Blondé E, Teodósio R, Araújo C, Pereira F. Health problems during and after travel: a prospective observational study in a travel clinic in Portugal. Acta Med Port. 2021;34(12):842–50. 10.20344/amp.14098. [DOI] [PubMed] [Google Scholar]
- 25.Rocklöv J, Quam MB, Sudre B, German M, Kraemer MUG, Brady O, Bogoch II, Liu-Helmersson J, Wilder-Smith A, Semenza JC et al. Assessing Seasonal Risks for the Introduction and Mosquito-borne Spread of Zika Virus in Europe. EBioMedicine 2016, 9:250-256.10.1016/j.ebiom.2016.06.009. [DOI] [PMC free article] [PubMed]
- 26.Sousa CA, Clairouin M, Seixas G, Viveiros B, Novo MT, Silva MT, Escoval AC, Economopoulou A. Ongoing outbreak of dengue type 1 in the Autonomous Region of Madeira, Portugal: preliminary report. Eurosurveillance. 2012;17(49). 10.2807/ese.17.49.20333-en. [DOI] [PubMed]
- 27.Wilder-Smith A, Quam M, Sessions O, Rocklov J, Liu-Helmersson J, Franco L, Khan K. The 2012 dengue outbreak in Madeira: exploring the origins. Eurosurveillance. 2014;19(8):20718. 10.2807/1560-7917.es2014.19.8.20718. [DOI] [PubMed] [Google Scholar]
- 28.Lourenço J. M Recker 2014 The 2012 Madeira Dengue Outbreak: epidemiological determinants and future epidemic potential. PLoS Negl Trop Dis 8 8 e3083 10.1371/journal.pntd.0003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lourenço J, Barros SC, Zé-Zé L, Damineli DSC, Giovanetti M, Osório HC, Amaro F, Henriques AM, Ramos F, Luís T et al. West Nile virus transmission potential in Portugal. Communications Biology 2022, 5(1).10.1038/s42003-021-02969-3. [DOI] [PMC free article] [PubMed]
- 30.Oliveira, DPd. Desenvolvimento De um sistema eletrónico de vigilância epidemiológica (eVDmed): recolha, análise e publicação de informação. Universidade do Minho; 2017.
- 31.Direção de Serviço de Informação e Análise DdEeV. Doenças de Declaração Obrigatória 2013–2016. In. Edited by Saúde D-Gd, vol. Volume I - Portugal. Lisboa; 2017.
- 32.Rede. de Vigilância de Vetores [https://www.insa.min-saude.pt/category/areas-de-atuacao/doencas-infeciosas/revive-rede-de-vigilancia-de-vetores/]
- 33.Vector-borne diseases [https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases]
- 34.ECDC: European Centre for Disease Prevention and Control.Organisation of vector surveillance and control in Europe. In. Stockholm. 2021.10.2900/853486.
- 35.Sistema Nacional de Vigilância Epidemiológica. [https://www.spms.min-saude.pt/2020/07/sinave-2/]
- 36.INE. Estatísticas da População - Estimativas: 2009–2019. In. Lisboa; 2022.
- 37.Almeida-Silva MB, Morais M, Morais I, Pena JNL, Pimenta P, Simão Márcia. Mariana.: 4th International Congress on Environmental Health In: NEW CHALLENGES FOR THE FUTURE -ABSTRACT PROCEEDINGS OF INTERNATIONAL CONGRESS ON ENVIRONMNETAL HEALTH 2019: 2019: Lisbon School of Health Technology Publisher; 2019.
- 38.WHO: World malaria report 2023. In: Global Malaria Programme (GMP). Edited by Organization WH. 2023: 283.
- 39.Paul RE, Diallo M, Brey PT. Mosquitoes and transmission of malaria parasites - not just vectors. Malar J 2004, 3:39.10.1186/1475-2875-3-39. [DOI] [PMC free article] [PubMed]
- 40.Lefevre T, Ohm J, Dabiré KR, Cohuet A, Choisy M, Thomas MB, Cator L. Transmission traits of malaria parasites within the mosquito: Genetic variation, phenotypic plasticity, and consequences for control. Evolutionary Applications 2018, 11(4):456-469.10.1111/eva.12571. [DOI] [PMC free article] [PubMed]
- 41.Moran PAP. The interpretation of statistical maps. J Royal Stat Soc Ser B (Methodological). 1948;10(2):243–51. [Google Scholar]
- 42.Anselin L. Local Indicators of Spatial Association—LISA. Geographical Analysis 1995, 27(2):93-115.10.1111/j.1538-4632.1995.tb00338.x.
- 43.Anselin L, Syabri I, Kho Y. GeoDa: An Introduction to Spatial Data Analysis. Geographical Analysis 2006, 38(1):5-22.10.1111/j.0016-7363.2005.00671.x.
- 44.Anselin L, Syabri I, Smirnov O. Visualizing Multivariate Spatial Correlation with Dynamically Linked Windows. New Tools for Spatial Data Analysis: Proceedings of the Specialist Meeting 2002.
- 45.Mann HB. Nonparametric Tests Against Trend. Econometrica 1945, 13(3):245-259.10.2307/1907187.
- 46.Kendall M, Gibbons JD. Rank Correlation Methods., 5th Edition edn. London; 1990.
- 47.Malária em Portugal. Memórias de uma Luta no Vale de Rio Sado [https://www.insa.min-saude.pt/malaria-em-portugal-memorias-de-uma-luta-no-vale-de-rio-sado/]
- 48.Lobo ARM. A História Da Malária em Portugal na Transição do Século IX para o Século XX e a Contribuição Da Escola De Medicina Tropical De Lisboa (1902–1935). Lisboa: Universidade Nova de Lisboa; 2012. [Google Scholar]
- 49.ECDC. Malaria - Annual Epidemiological Report for 2019. In. European Centre for Disease Prevention and Control; 2021.
- 50.Silva NM, Santos NC, Martins IC. Dengue and Zika Viruses: Epidemiological History, Potential Therapies, and Promising Vaccines. Tropical Medicine and Infectious Disease 2020, 5(4):150.10.3390/tropicalmed5040150. [DOI] [PMC free article] [PubMed]
- 51.Manica M, Marini G, Solimini A, Guzzetta G, Poletti P, Scognamiglio P, Virgillito C, Della Torre A, Merler S, Rosà R et al. Reporting delays of chikungunya cases during the 2017 outbreak in Lazio region, Italy. PLOS Neglected Tropical Diseases 2023, 17(9):e0011610.10.1371/journal.pntd.0011610. [DOI] [PMC free article] [PubMed]
- 52.Zé-Zé L. Zika virus infections imported from Brazil to Portugal, 2015. IDCases 2016, 4:46-49.10.1016/j.idcr.2016.03.004. [DOI] [PMC free article] [PubMed]
- 53.Oliveira S, Capinha C, Rocha J. Predicting the time of arrival of the Tiger mosquito Aedes albopictus to new countries based on trade patterns of tyres and plants. Journal of Applied Ecology 2023, 60(11):2362-2374.10.1111/1365-2664.14503.
- 54.Massad E, Tan S-H, Khan K, Wilder-Smith A. Estimated Zika virus importations to Europe by travellers from Brazil. Global Health Action 2016, 9(1):31669.10.3402/gha.v9.31669. [DOI] [PMC free article] [PubMed]
- 55.Shi Z, Pun-Cheng L. Spatiotemporal Data Clustering: A Survey of Methods. ISPRS International Journal of Geo-Information 2019, 8(3):112.10.3390/ijgi8030112.
- 56.Sugumaran R, Larson SR, Degroote JP. Spatio-temporal cluster analysis of county-based human West Nile virus incidence in the continental United States. International Journal of Health Geographics 2009, 8(1):43.10.1186/1476-072x-8-43. [DOI] [PMC free article] [PubMed]
- 57.Stewart-Ibarra AM, Muñoz ÁG, Ryan SJ, Ayala EB, Borbor-Cordova MJ, Finkelstein JL, Mejía R, Ordoñez T, Recalde-Coronel GC, Rivero K. Spatiotemporal clustering, climate periodicity, and social-ecological risk factors for dengue during an outbreak in Machala, Ecuador, in 2010. BMC Infectious Diseases 2014, 14(1).10.1186/s12879-014-0610-4. [DOI] [PMC free article] [PubMed]
- 58.Wu X, Hu S, Kwaku AB, Li Q, Luo K, Zhou Y, Tan H. Spatio-temporal clustering analysis and its determinants of hand, foot and mouth disease in Hunan, China, 2009–2015. BMC Infectious Diseases 2017, 17(1).10.1186/s12879-017-2742-9. [DOI] [PMC free article] [PubMed]
- 59.Bertola M, Fornasiero D, Sgubin S, Mazzon L, Pombi M, Montarsi F. Comparative efficacy of BG-Sentinel 2 and CDC-like mosquito traps for monitoring potential malaria vectors in Europe. Parasites & Vectors 2022, 15(1).10.1186/s13071-022-05285-9. [DOI] [PMC free article] [PubMed]
- 60.Wilke ABB, Vasquez C, Carvajal A, Moreno M, Petrie WD, Beier JC. Evaluation of the effectiveness of BG-Sentinel and CDC light traps in assessing the abundance, richness, and community composition of mosquitoes in rural and natural areas. Parasites & Vectors 2022, 15(1).10.1186/s13071-022-05172-3. [DOI] [PMC free article] [PubMed]
- 61.Brooks M. The Advantages of Comparative LISA Techniques in Spatial Inequality Research: Evidence from Poverty Change in the United States. Spatial Demography 2019, 7.10.1007/s40980-019-00052-4.
- 62.Bonnet E, Fournet F, Benmarhnia T, Ouedraogo S, Dabiré R, Ridde V. Impact of a community-based intervention on Aedes aegypti and its spatial distribution in Ouagadougou, Burkina Faso. Infect Dis Poverty 2020, 9(1):61.10.1186/s40249-020-00675-6. [DOI] [PMC free article] [PubMed]
- 63.Chen X, Wang H, Lyu W, Xu R. The Mann-Kendall-Sneyers test to identify the change points of COVID-19 time series in the United States. BMC Medical Research Methodology 2022, 22(1).10.1186/s12874-022-01714-6. [DOI] [PMC free article] [PubMed]
- 64.Guo M, Li J, He H, Xu J, Jin YH. Detecting Global Vegetation Changes Using Mann-Kendal (MK) Trend Test for 1982–2015 Time Period. Chinese Geographical Science 2018, 28:907-919.10.1007/s11769-018-1002-2.
- 65.Jones RT, Ant TH, Cameron MM, Logan JG. Novel control strategies for mosquito-borne diseases. Philosophical Transactions of the Royal Society B: Biological Sciences 2021, 376(1818):20190802.10.1098/rstb.2019.0802. [DOI] [PMC free article] [PubMed]
- 66.Petersen LR, Beard CB, Visser SN. Combatting the Increasing Threat of Vector-Borne Disease in the United States with a National Vector-Borne Disease Prevention and Control System. The American Journal of Tropical Medicine and Hygiene 2019, 100(2):242-245.10.4269/ajtmh.18–0841. [DOI] [PMC free article] [PubMed]
- 67.Rocklöv J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nature Immunology 2020, 21(5):479-483.10.1038/s41590-020-0648-y. [DOI] [PMC free article] [PubMed]
- 68.Milano A, Robbiati C, Declich S, Calistri P, Pediconi O, Amato L, Paronyan L, Avetisyan L, Manucharyan A, Avetisyan G et al. Assessing the Adoption of One Health Approaches in National Plans to Combat Health Threats: The Pilot of a One Health Conceptual Framework in Armenia. Tropical Medicine and Infectious Disease 2024, 9(1):22.10.3390/tropicalmed9010022. [DOI] [PMC free article] [PubMed]
- 69.Millet JP, Montalvo T, Bueno-Marí R, Romero-Tamarit A, Prats-Uribe A, Fernández L, Camprubí E, del Baño L, Peracho V, Figuerola J et al. Imported zika virus in a European city: How to prevent local transmission? Frontiers in Microbiology 2017, 8(JUL).10.3389/fmicb.2017.01319. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Statistics Portugal datasets on population are available online at Statistics Portugal website (https://www.ine.pt/) The SINAVE and REVIVE datasets can be obtained upon formal request to the respective entities.