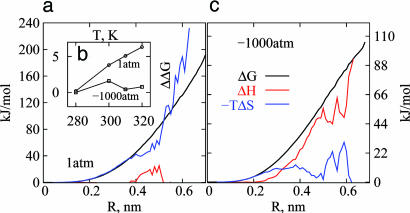
Fig. 4.
Thermodynamics of small and large solute hydration. (a and c) The hydration free energy of a hard-sphere solute, ΔG, as a function of solute size R, at 1 atm (a) and -1,000 atm (c) at 300 K. (b) The temperature dependence of ΔG is shown with reference to its value at 280 K [ΔΔG = ΔG(T) - ΔG(T = 280K)] for a hard-sphere solute of radius 4.5 Å. (c) The enthalpic (ΔH, red) and entropic (-TΔS, blue) contributions to ΔG obtained from temperature derivatives of the free energy are shown at 300 K. Thermodynamic crossover from entropic to enthalpic hydration is clear at -1,000 atm.