Abstract
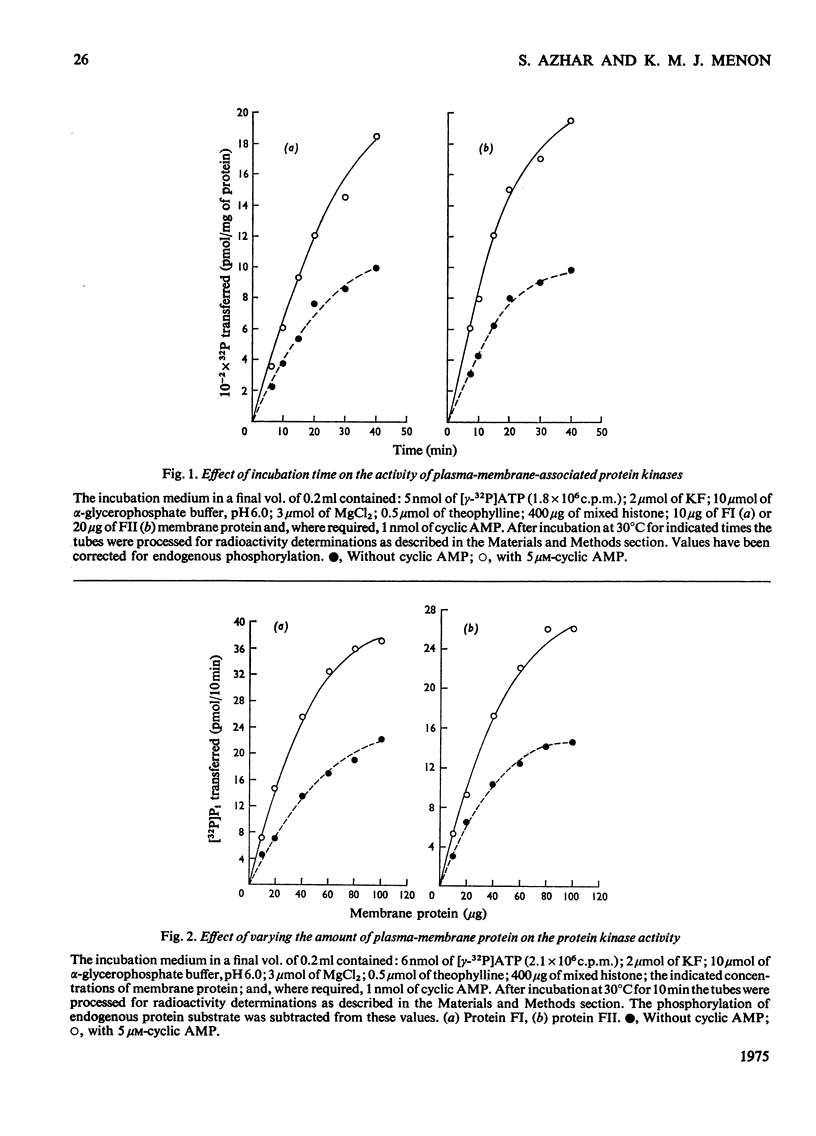
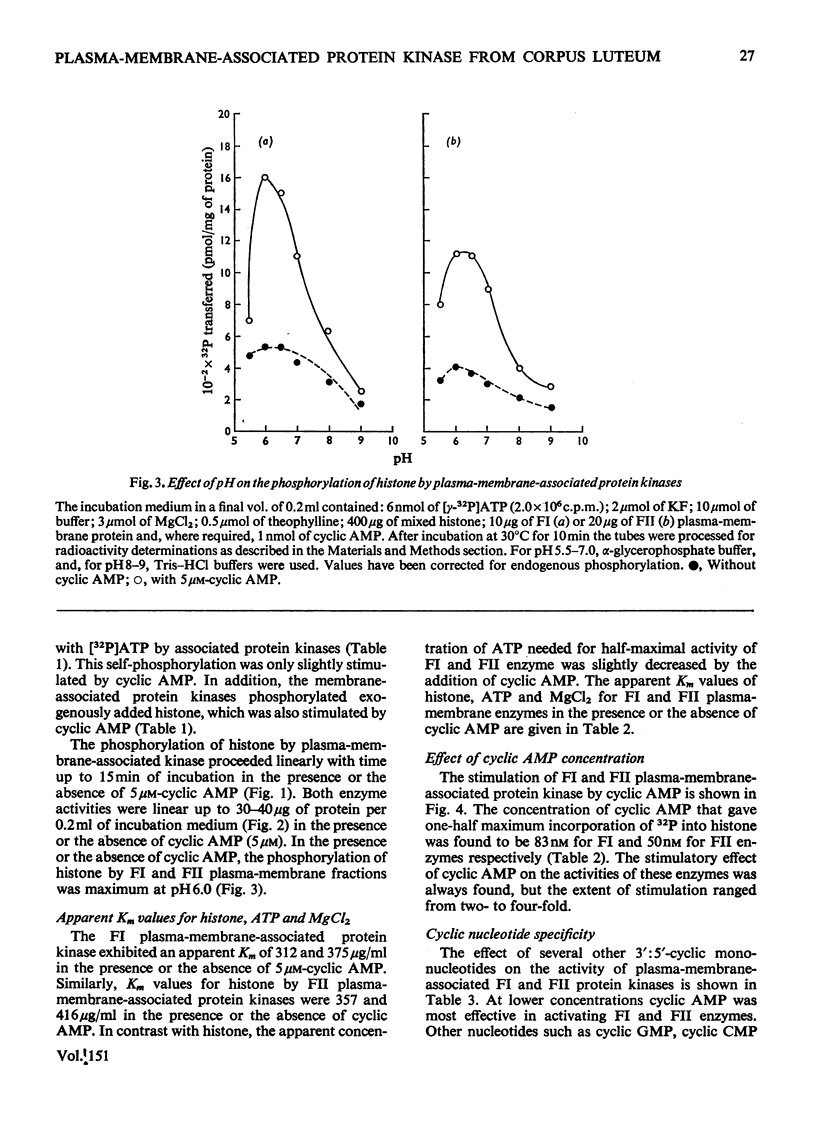
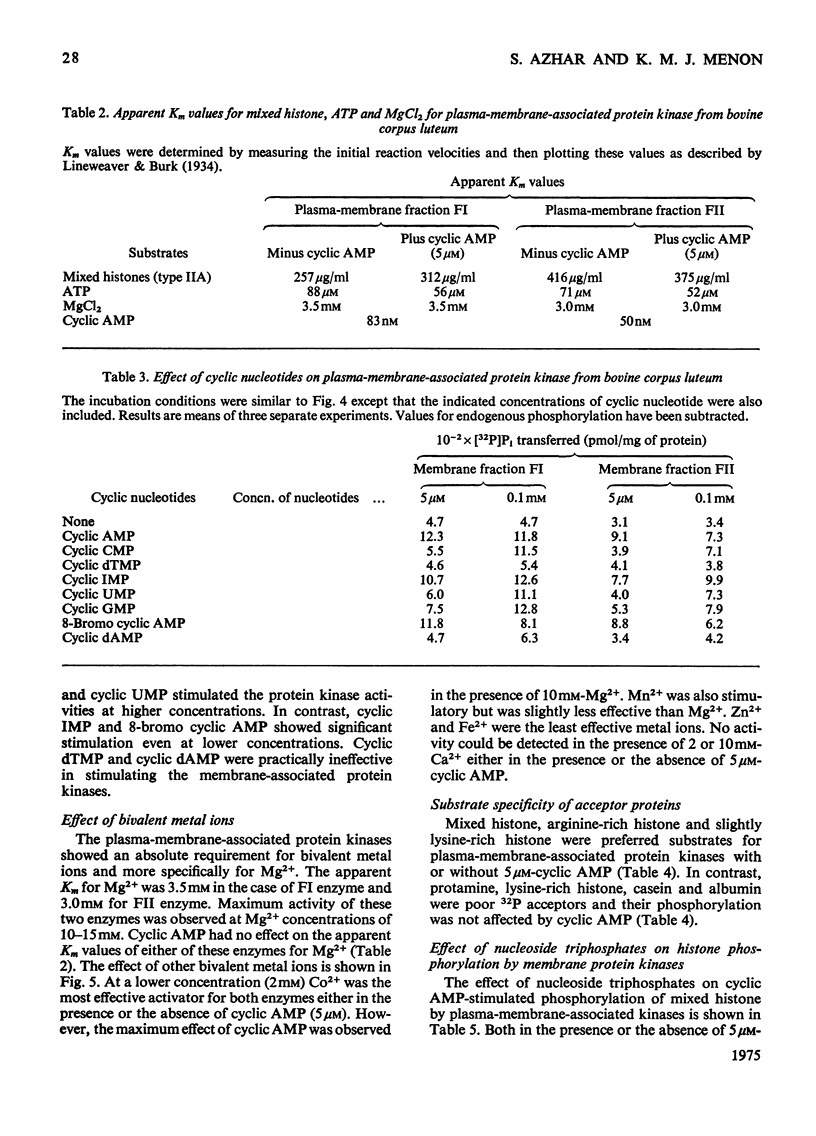
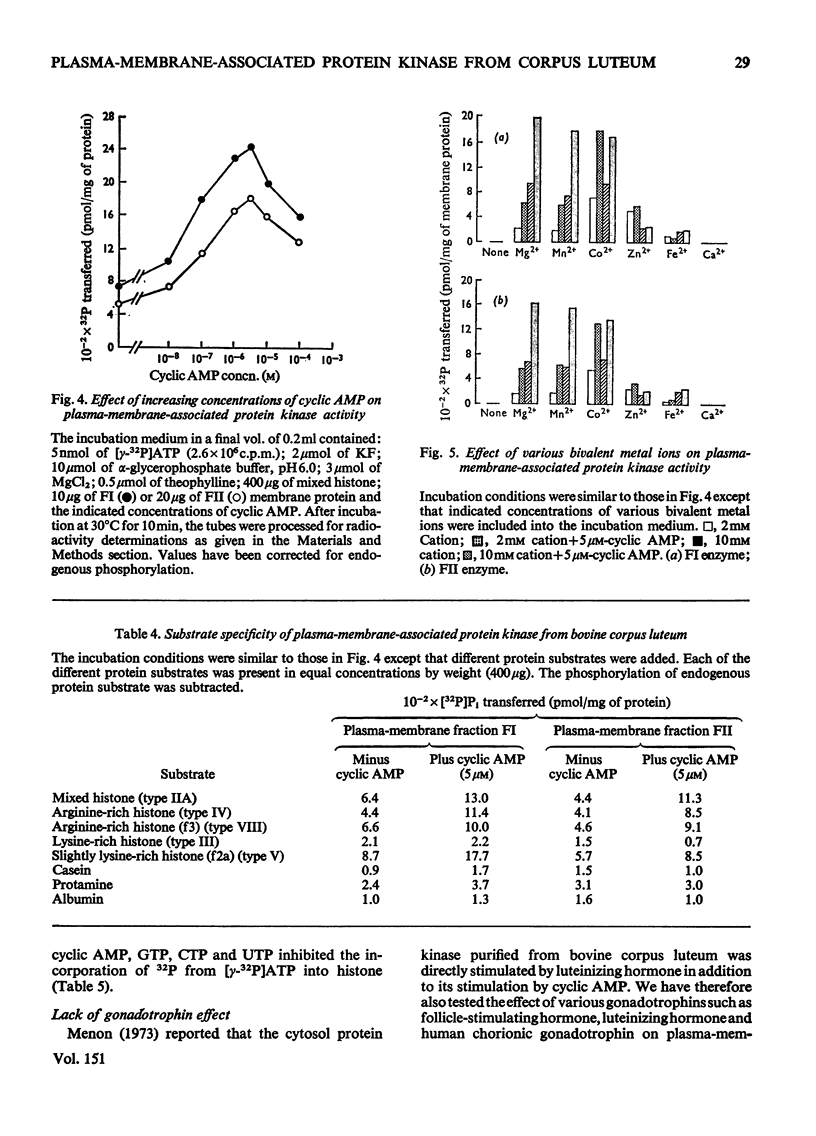
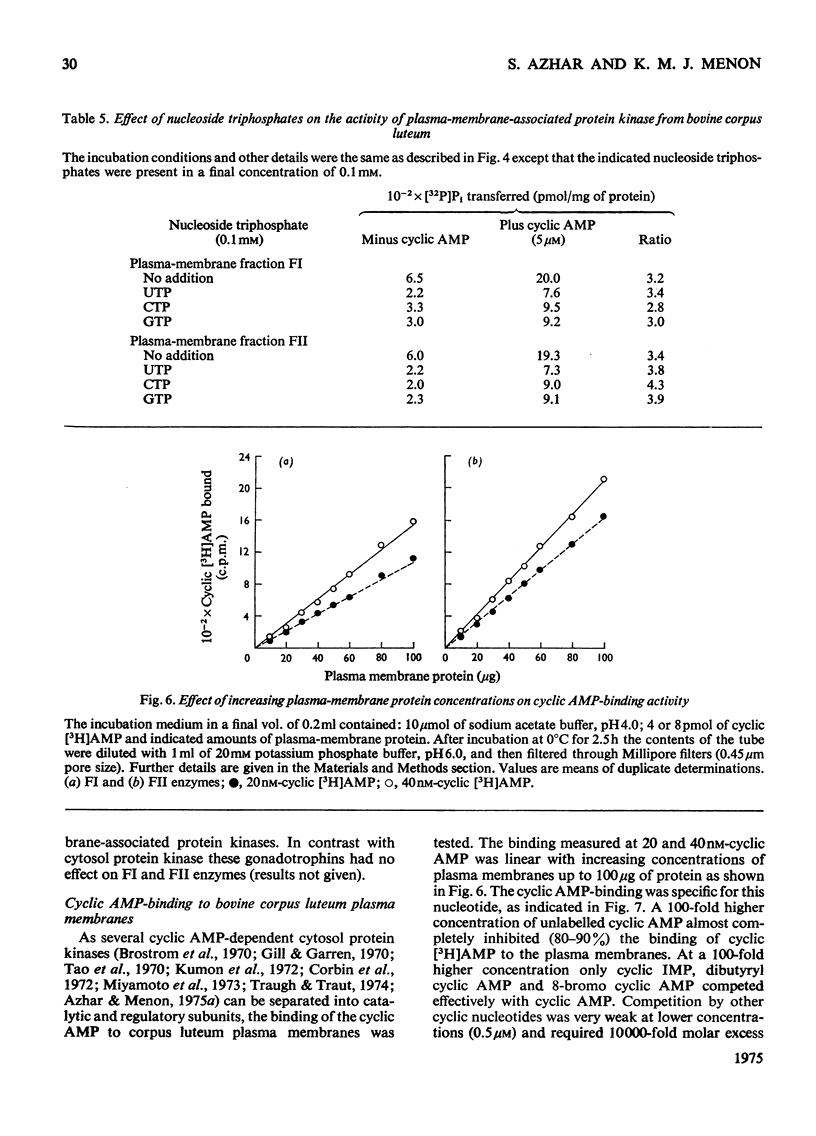
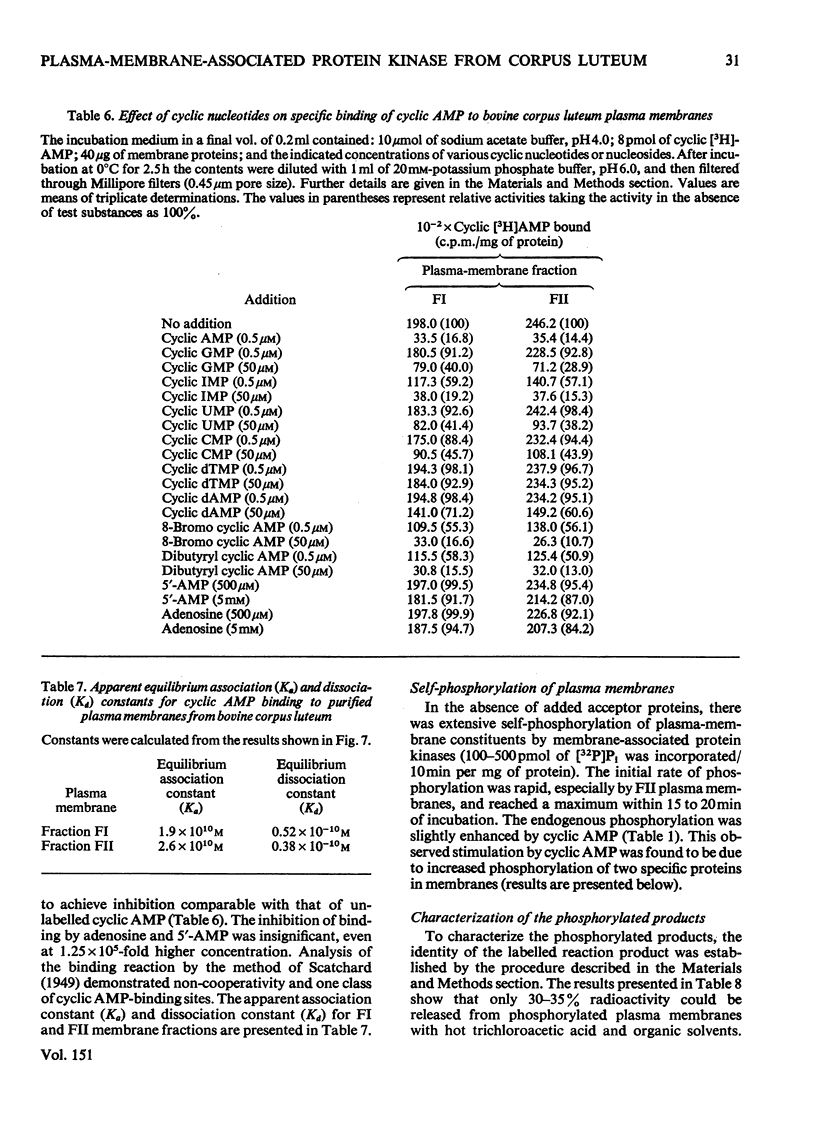
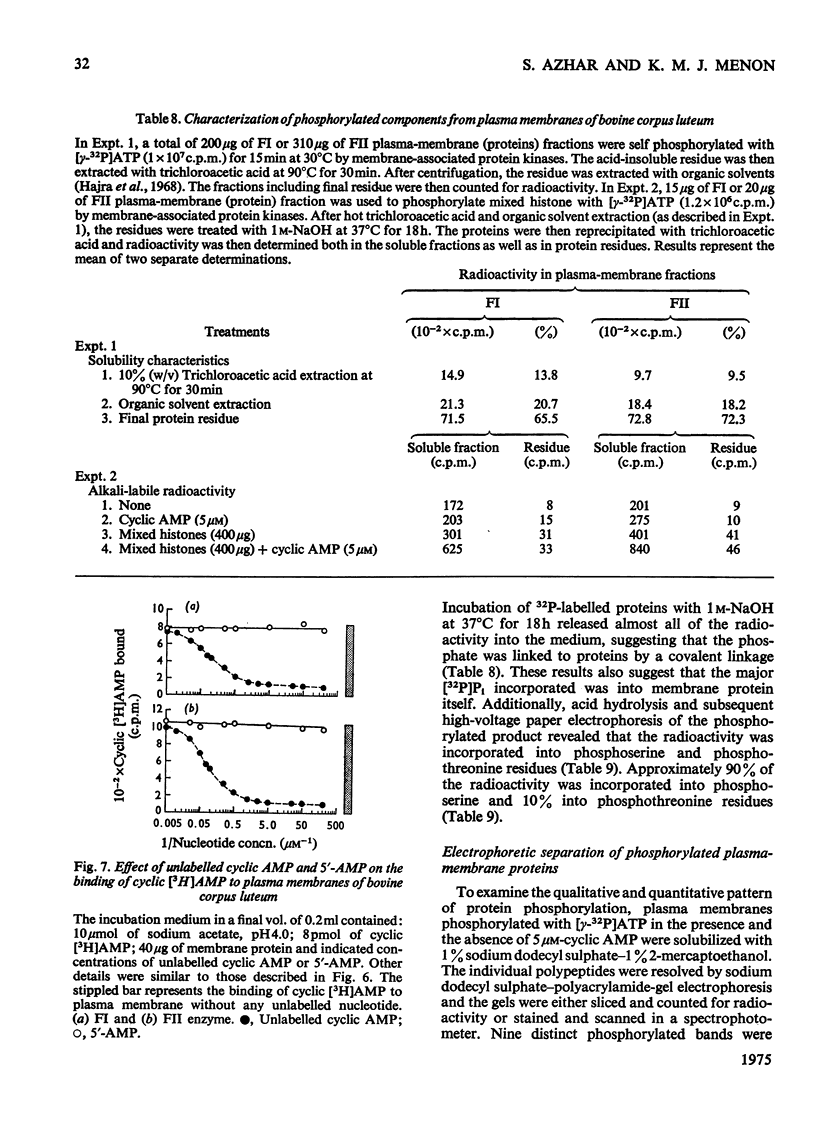
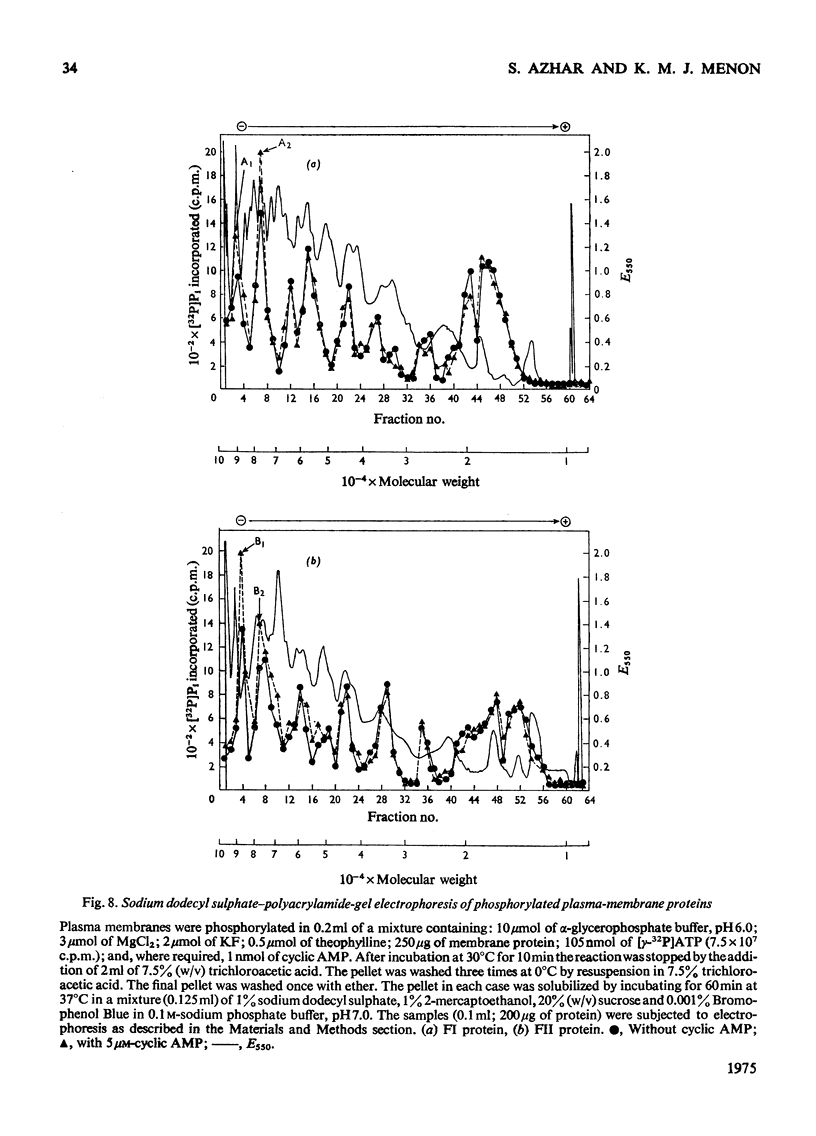
Plasma-membrane fractions FI and FII isolated from bovine corpus luteum by discontinuous sucrose-density-gradient centrifugation, at sucrose-density interfaces of 1.14/1.16 and 1.16/1.18 respectively, contained membrane-associated protein kinases that phosphorylated both the structural proteins of membranes as well as exogenously added protein substrates. Both fractions were characterized with respect to endogenous and exogenous protein substrate specificity, pH-dependence, effect of bivalent metal ions and sensitivity toward cyclic nucleotides. These membrane-associated kinases showed an optimum pH of 6.0 and had an absolute requirement for bivalent metal ions such as Mg2+, Mn2+, or Co2+ that cannot be replaced by Ca2+. Both the activities were stimulated two- to four-fold by cyclic AMP in vitro with an apparent Km of 83 and 50 nM for fractions FI and FII respectively. Other cyclic 3':5'-nucleotides were effective only at higher concentrations, but even the most effective, cyclic IMP, showed a stimulation nearly an order of magnitude lower than that of cyclic AMP. In contrast, stimulation by cyclic dTMP and cyclic dAMP was very weak. Cyclic AMP showed no significant effect on the apparent Km value of both enzymes for histone and MgCl2 but it somewhat decreased the Km value for ATP. Nucleoside triphosphates like GTP, CTP and UTP inhibited the transfer of [32P]Pi from [gamma-32P]ATP into mixed histone catalysed by membrane-associated kinases either in the presence or in the absence of cyclic AMP. In addition to protein kinases, these membrane fractions also possessed cyclic AMP-binding activities. The apparent association constant (Kalpha) for cyclic AMP binding was 1.0 X 10(10) and 2.6 X 10(10) M for FI and FII membrane fractions respectively.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azhar S., Menon K. M. Adenosine 3'5'-m onophosphate dependent phosphorylation of ribosomes and ribosomal subunits from bovine corpus luteum. Biochim Biophys Acta. 1975 May 5;392(1):64–74. doi: 10.1016/0304-4165(75)90166-x. [DOI] [PubMed] [Google Scholar]
- Azhar S., Menon K. M. Cyclic adenosine 3',5'-monophosphate and luteinizing hormone stimulated protein kinase from bovine corpus luteum: evidence for activation through separate mechanisms. FEBS Lett. 1975 Mar 1;51(1):25–28. doi: 10.1016/0014-5793(75)80847-7. [DOI] [PubMed] [Google Scholar]
- Bacalao J., Rieber M. On the properties of a membrane-associated protein kinase from Chinese hamster ovary cells. FEBS Lett. 1973 Nov 15;37(1):37–41. doi: 10.1016/0014-5793(73)80421-1. [DOI] [PubMed] [Google Scholar]
- Brostrom M. A., Reimann E. M., Walsh D. A., Krebs E. G. A cyclic 3',5'-amp-stimulated protein kinase from cardiac muscle. Adv Enzyme Regul. 1970;8:191–203. doi: 10.1016/0065-2571(70)90017-8. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Chang K. J., Marcus N. A., Cuatrecasas P. Cyclic adenosine monophosphate-dependent phosphorylation of specific fat cell membrane proteins by an endogenous membrane-bound protein kinase. Possible involvement in the regulation of insulin-stimulated glucose transport. J Biol Chem. 1974 Nov 10;249(21):6854–6865. [PubMed] [Google Scholar]
- Corbin J. D., Brostrom C. O., King C. A., Krebs E. G. Studies on the adenosine 3',5'-monophosphate-dependent protein kinases of rabbit skeletal muscle. J Biol Chem. 1972 Dec 10;247(23):7790–7798. [PubMed] [Google Scholar]
- Corbin J. D., Krebs E. G. A cyclic AMP--stimulated protein kinase in adipose tissue. Biochem Biophys Res Commun. 1969 Jul 23;36(2):328–336. doi: 10.1016/0006-291x(69)90334-9. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Walton K. G., Curran P. F., Greengard P. Regulation of phosphorylation of a specific protein in toad-bladder membrane by antidiuretic hormone and cyclic AMP, and its possible relationship to membrane permeability changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):880–884. doi: 10.1073/pnas.70.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. A cyclic-3',5'-adenosine monophosphate dependent protein kinase from the adrenal cortex: comparison with a cyclic AMP binding protein. Biochem Biophys Res Commun. 1970 May 11;39(3):335–343. doi: 10.1016/0006-291x(70)90581-4. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Preparation and characterization of plasma membranes from bovine corpus luteum. J Biol Chem. 1973 Jul 25;248(14):5050–5056. [PubMed] [Google Scholar]
- Guthrow C. E., Jr, Allen J. E., Rasmussen H. Phosphorylation of an endogenous membrane protein by an endogenous, membrane-associated cyclic adenosine 3',5'-monophosphate-dependent protein kinase in human erythrocyte ghosts. J Biol Chem. 1972 Dec 25;247(24):8145–8153. [PubMed] [Google Scholar]
- Hajra A. K., Seguin E. B., Agranoff B. W. Rapid labeling of mitochondrial lipids by labeled orthophosphate and adenosine triphosphate. J Biol Chem. 1968 Apr 10;243(7):1609–1616. [PubMed] [Google Scholar]
- Hokin L. E., Sastry P. S., Galsworthy P. R., Yoda A. Evidence that a phosphorylated intermediate in a brain transport adenosine triphosphatase is an acyl phosphate. Proc Natl Acad Sci U S A. 1965 Jul;54(1):177–184. doi: 10.1073/pnas.54.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jard S., Bastide F. A cyclic AMP-dependent protein kinase from frog bladder epithelial cells. Biochem Biophys Res Commun. 1970 May 22;39(4):559–566. doi: 10.1016/0006-291x(70)90240-8. [DOI] [PubMed] [Google Scholar]
- Jergil B., Dixon G. H. Protamine kinase from rainbow trout testis. Partial purification and characterization. J Biol Chem. 1970 Jan 25;245(2):425–434. [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. Characterization and regulatory aspects. Biochemistry. 1970 Oct 13;9(21):4160–4175. doi: 10.1021/bi00823a019. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- Kumon A., Nishiyama K., Yamamura H., Nishizuka Y. Multiplicity of adenosine 3',5'-monophosphate-dependent protein kinases from rat liver and mode of action of nucleoside 3',5'-monophosphate. J Biol Chem. 1972 Jun 25;247(12):3726–3735. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Action of adenosine 3',5'-monophosphate-dependent histone kinase in vivo. J Biol Chem. 1969 Oct 25;244(20):5763–5765. [PubMed] [Google Scholar]
- Langan T. A. Phosphorylation of liver histone following the administration of glucagon and insulin. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1276–1283. doi: 10.1073/pnas.64.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie C. W., 3rd, Stellwagen R. H. Heterogeneity and unusually high affinity in the interactions of adenosine 3':5'-monophosphate with specific binding proteins from liver and hepatoma cells. J Biol Chem. 1974 Sep 25;249(18):5763–5771. [PubMed] [Google Scholar]
- Marsh J. M., Butcher R. W., Savard K., Sutherland E. W. The stimulatory effect of luteinizing hormone on adenosine 3',5'-monophosphate accumulation in corpus luteum slices. J Biol Chem. 1966 Nov 25;241(22):5436–5440. [PubMed] [Google Scholar]
- Marsh J. M., Savard K. The stimulation of progesterone synthesis in bovine corpora lutea by adenosine 3',5'-monophosphate. Steroids. 1966 Aug;8(2):133–148. doi: 10.1016/0039-128x(66)90088-2. [DOI] [PubMed] [Google Scholar]
- Marsh J. M. The stimulatory effect of luteinizing hormone on adenyl cyclase in the bovine corpus luteum. J Biol Chem. 1970 Apr 10;245(7):1596–1603. [PubMed] [Google Scholar]
- Menon K. M., Kiburz J. Isolation of plasma membranes from bovine corpus luteum possessing adenylate cyclase, 125I-hCG binding and Na-K-ATPase activities. Biochem Biophys Res Commun. 1974 Jan 23;56(2):363–371. doi: 10.1016/0006-291x(74)90851-1. [DOI] [PubMed] [Google Scholar]
- Menon K. M. Purification and properties of a protein kinase from bovine corpus luteum that is stimulated by cyclic adenosine 3',5'-monophosphate and luteinizing hormone. J Biol Chem. 1973 Jan 25;248(2):494–501. [PubMed] [Google Scholar]
- Miyamoto E., Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. 3. Purification and properties of adenosine 3',5'-monophosphate-dependent protein kinase from bovine brain. J Biol Chem. 1969 Dec 10;244(23):6395–6402. [PubMed] [Google Scholar]
- Miyamoto E., Petzold G. L., Kuo J. F., Greengard P. Dissociation and activation of adenosine 3',5'-monophosphate-dependent and guanosine 3',5'-monophosphate-dependent protein kinases by cyclic nucleotides and by substrate proteins. J Biol Chem. 1973 Jan 10;248(1):179–189. [PubMed] [Google Scholar]
- Najjar V. A., Constantopoulos A. The activation of adenylate cyclase. I. A postulated mechanism for fluoride and hormone activation of adenylate cyclase. Mol Cell Biochem. 1973 Nov 15;2(1):87–93. doi: 10.1007/BF01738682. [DOI] [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- RAPKIN E. LIQUID SCINTILLATION COUNTING 1957--1963. A REVIEW. Int J Appl Radiat Isot. 1964 Feb;15:69–87. doi: 10.1016/0020-708x(64)90052-3. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Rieber M., Bacalao J. Phosphorylation of high-molecular-weight membrane protein species in Chinese-hamster ovary cells in culture: effect of 6-N,2'-O-dibutyryladenosine 3':5'-cyclic monophosphate plus testosterone. Biochem J. 1973 Mar;132(3):641–644. doi: 10.1042/bj1320641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses A. D., Appel S. H. Erythrocyte protein phosphorylation. J Biol Chem. 1973 Feb 25;248(4):1408–1411. [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Molecular forms and subunit composition of a cyclic adenosine 3',5'-monophosphate-dependent protein kinase purified from bovine heart muscle. J Biol Chem. 1972 Jan 10;247(1):36–44. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. The role of cyclic AMP in the phosphorylation of proteins in human erythrocyte membranes. Biochem Biophys Res Commun. 1973 Jan 23;50(2):421–429. doi: 10.1016/0006-291x(73)90857-7. [DOI] [PubMed] [Google Scholar]
- Russell T. R., Pastan I. H. Cyclic adenosine 3':5'-monophosphate and cyclic guanosine 3':5'-monophosphate phosphodiesterase activities are under separate genetic control. J Biol Chem. 1974 Dec 25;249(24):7764–7769. [PubMed] [Google Scholar]
- SAVARD K., MARSH J. M., RICE B. F. GONADOTROPINS AND OVARIAN STEROIDOGENESIS. Recent Prog Horm Res. 1965;21:285–365. [PubMed] [Google Scholar]
- Solyom A., Trams E. G. Enzyme markers in characterization of isolated plasma membranes. Enzyme. 1972;13(5-6):329–372. doi: 10.1159/000459682. [DOI] [PubMed] [Google Scholar]
- Tao M., Salas M. L., Lipmann F. Mechanism of activation by adenosine 3':5'-cyclic monophosphate of a protein phosphokinase from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Sep;67(1):408–414. doi: 10.1073/pnas.67.1.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Maeno H., Greengard P. Regulation of endogenous phosphorylation of specific proteins in synaptic membrane fractions from rat brain by adenosine 3':5'-monophosphate. J Biol Chem. 1973 Dec 10;248(23):8295–8305. [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


