Abstract
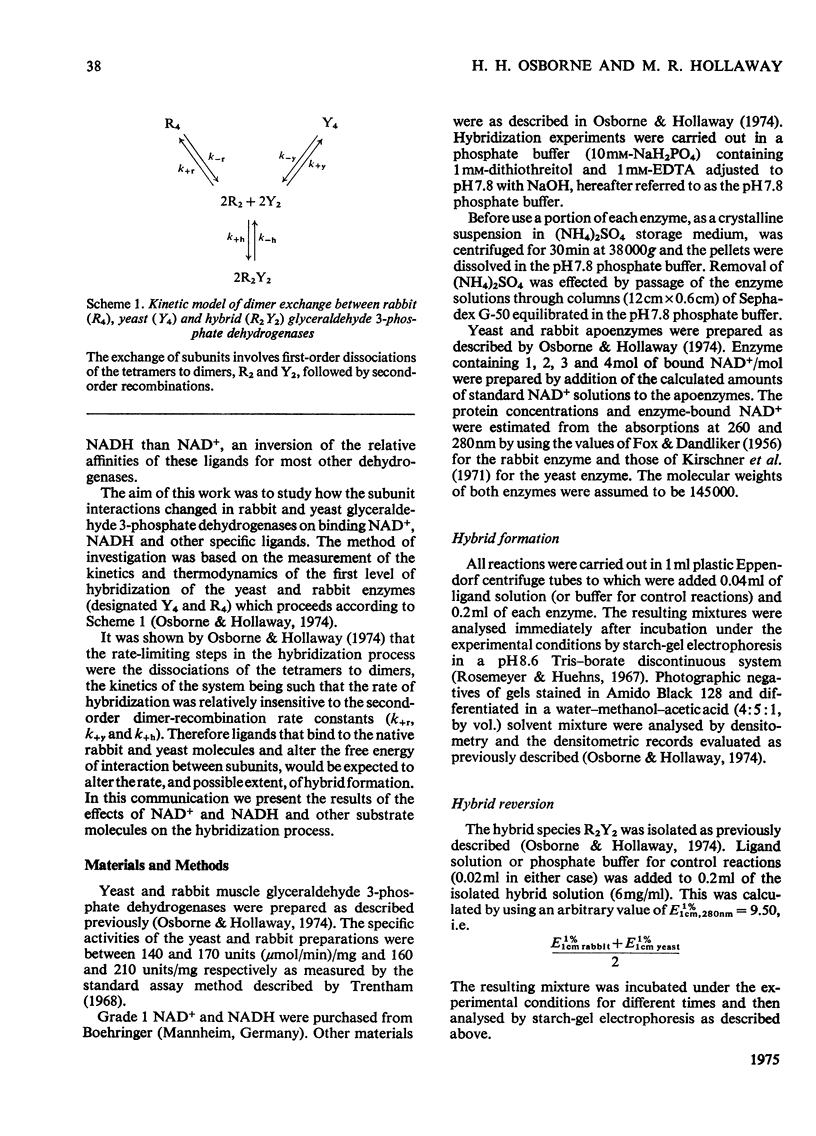
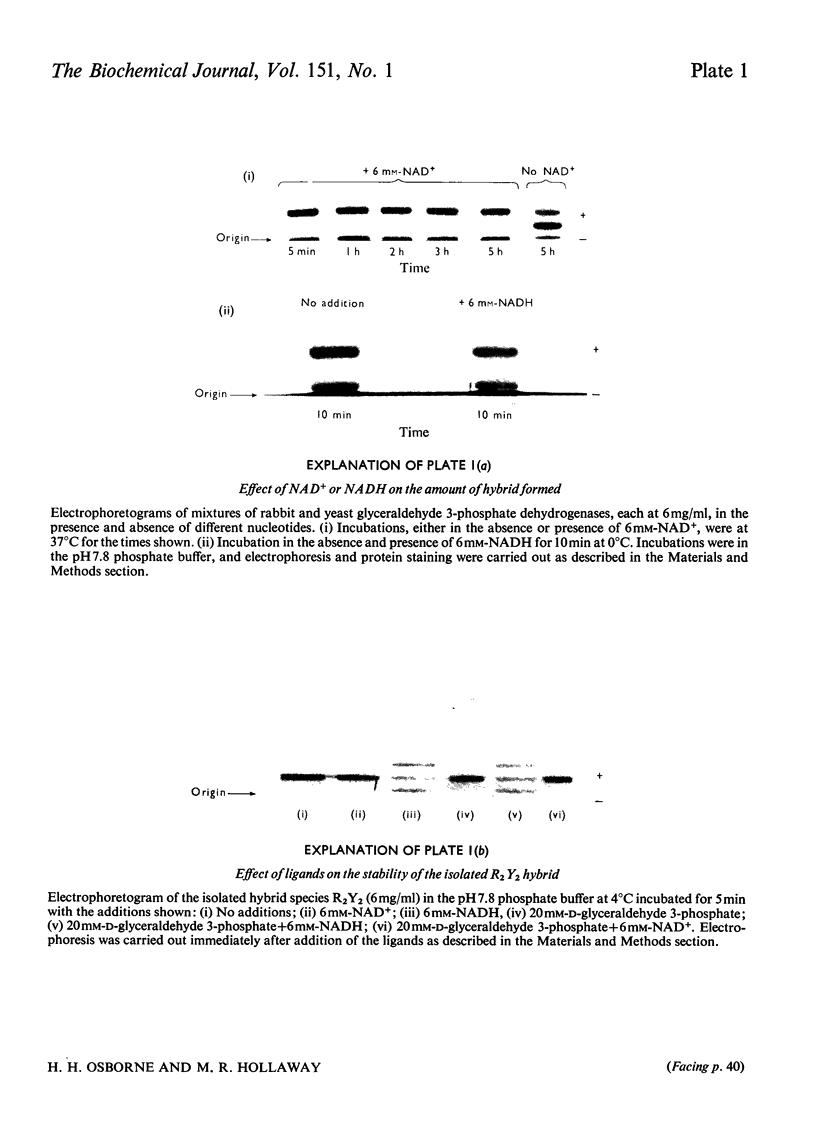
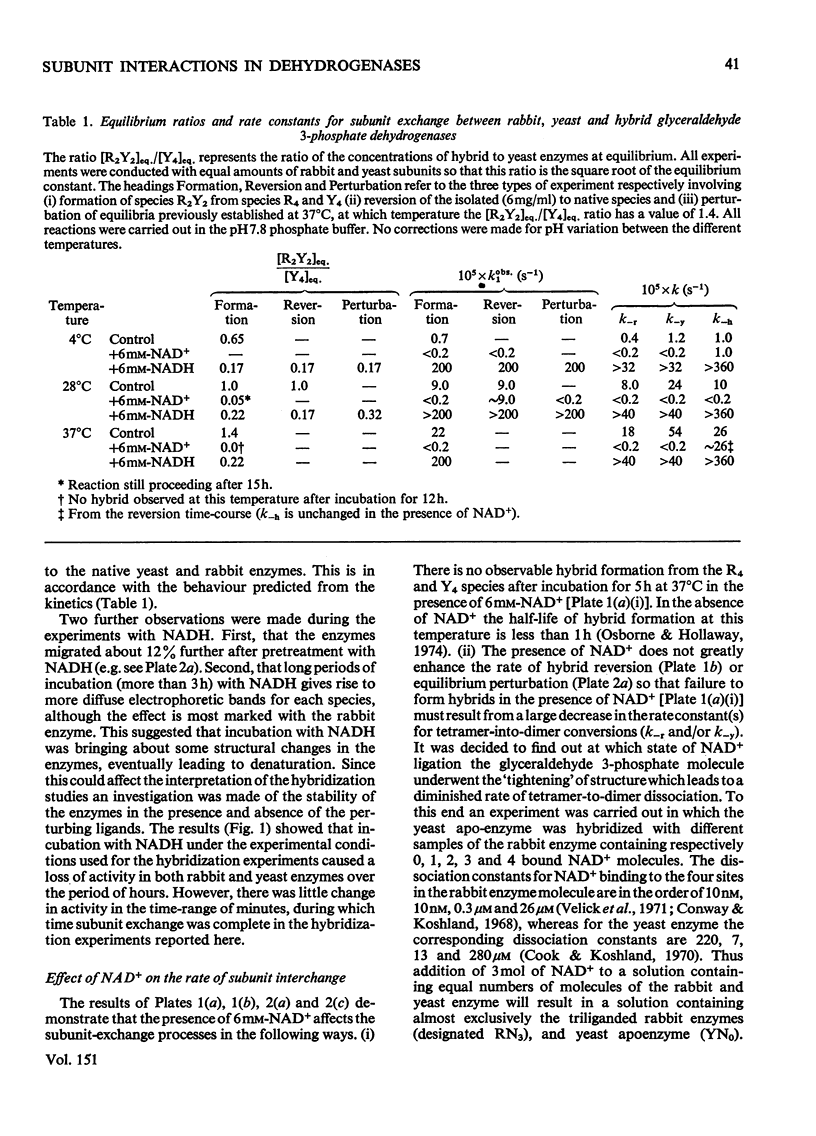
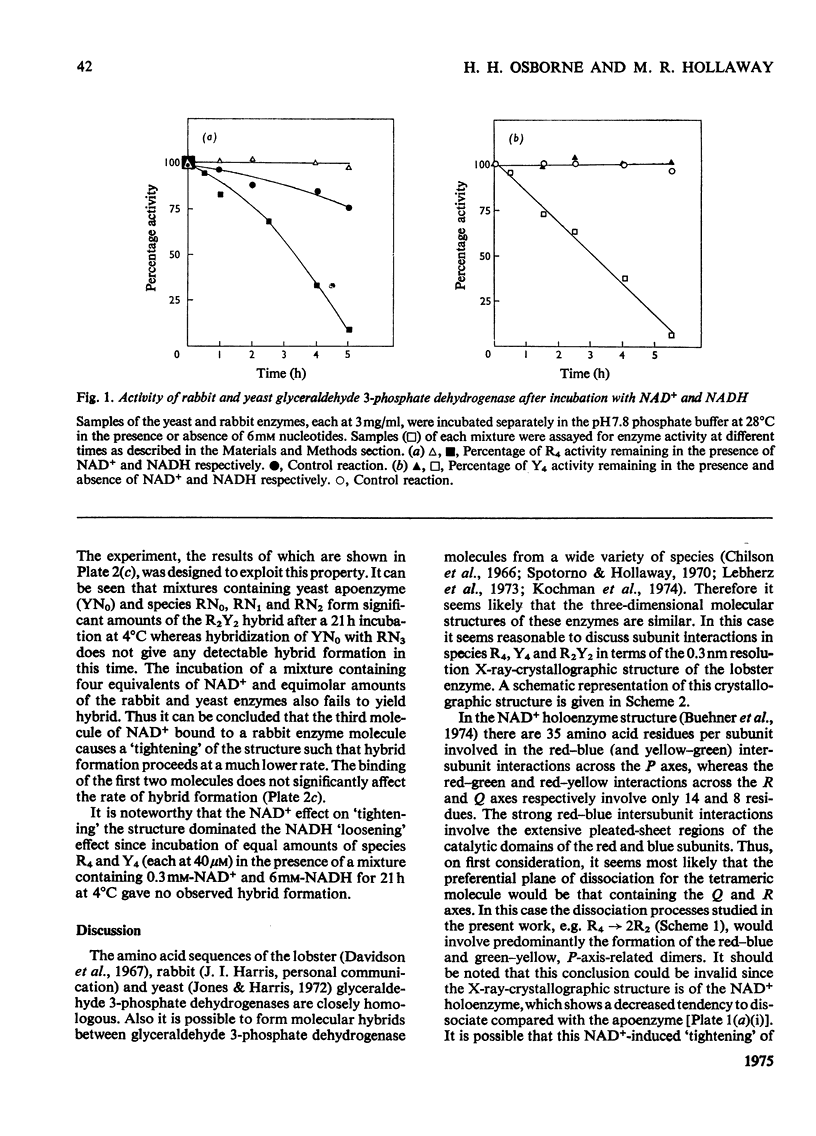
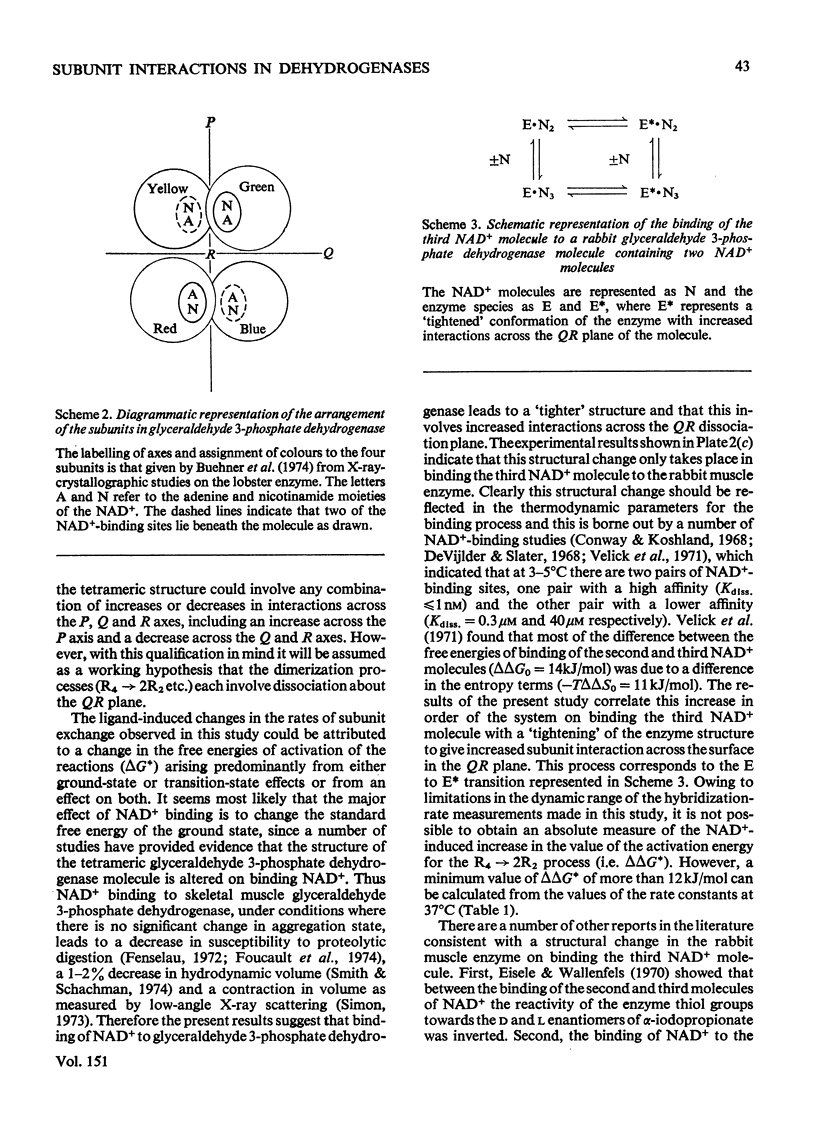
An investigation was made of changes in subunit interactions in glyceraldehyde 3-phosphate dehydrogenase on binding NAD+, NADH and other substrates by using the previously developed method of measurement of rates and extent of subunit exchange between the rabbit enzyme (R4), yeast enzyme (Y4) and rabbit-yeast hybrid (R2Y2) [Osborne & Hollaway (1974) Biochem. J. 143, 651-662]. The free energy of activation for the conversion of tetramer into dimer for the rabbit enzyme (R4 leads to 2R2) is increased by at least 12kJ/mol in the presence of NAD+. This increase is interpreted in terms of an NAD+-induced 'tightening' of the tetrameric structure probably involving increased interaction at the subunit interfaces across the QR plane of the molecule [see Buehner et al. (1974) J. Mol. Biol. 82, 563-585]. This tightening of the structure only occurs on binding the third NAD+ molecule to a given enzyme molecule. Conversely, binding of NADH causes a decrease in the free energy of activation for the R4 leads to 2R2 and Y4 leads to 2Y2 conversions by at least 10kJ/mol. This is interpreted as a NADH-induced 'loosening' of the structures arising from decreased interactions across the subunit interfaces involving the QR dissociation plane. In the presence of NADH the increase in the rate of subunit exchange is such that it is not possible to separate the hybrid from the other species if electrophoresis is carried out with NADH in the separation media. In the presence of a mixture of NADH and NAD+ the effect of NAD+ on subunit exchange is dominant. The results are discussed in terms of the known co-operativty between binding sites in glyceraldehyde 3-phosphate dehydrogenases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boers W., Oosthuizen C., Slater E. C. Binding of NAD + and NADH to rabbit-muscle glyceraldehydephosphate dehydrogenase. Biochim Biophys Acta. 1971 Oct;250(1):35–46. doi: 10.1016/0005-2744(71)90117-3. [DOI] [PubMed] [Google Scholar]
- Buehner M., Ford G. C., Olsen K. W., Moras D., Rossman M. G. Three-dimensional structure of D-glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1974 Nov 25;90(1):25–49. doi: 10.1016/0022-2836(74)90254-x. [DOI] [PubMed] [Google Scholar]
- Chilson O. P., Kitto G. B., Pudles J., Kaplan N. O. Reversible inactivation of dehydrogenases. J Biol Chem. 1966 May 25;241(10):2431–2445. [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- Cook R. A., Koshland D. E., Jr Positive and negative cooperativity in yeast glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1970 Aug 18;9(17):3337–3342. doi: 10.1021/bi00819a007. [DOI] [PubMed] [Google Scholar]
- DANDLIKER W. B., FOX J. B., Jr The coenzyme content of rabbit muscle D-glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1956 Aug;221(2):1005–1017. [PubMed] [Google Scholar]
- Davidson B. E., Sajgò M., Noller H. F., Harris J. I. Amino-acid sequence of glyceraldehyde 3-phosphate dehydrogenase from lobster muscle. Nature. 1967 Dec 23;216(5121):1181–1185. doi: 10.1038/2161181a0. [DOI] [PubMed] [Google Scholar]
- Fensleau A. Structure-function studies on glyceraldehyde 3-phosphate dehydrogenase. IV. Subunit interactions of the rabbit muscle and yeast enzymes. J Biol Chem. 1972 Feb 25;247(4):1074–1079. [PubMed] [Google Scholar]
- Foucault G., Traore F., Levilliers J., Pudles J. Structure-function relationship in rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Trinitrophenylation of the lysine residues. Eur J Biochem. 1974 Jul 1;46(1):43–57. doi: 10.1111/j.1432-1033.1974.tb03595.x. [DOI] [PubMed] [Google Scholar]
- Harris J. I., Perham R. N. Glyceraldehyde 3-phosphate dehydrogenase from pig muscle. Nature. 1968 Sep 7;219(5158):1025–1028. doi: 10.1038/2191025a0. [DOI] [PubMed] [Google Scholar]
- Jones G. M.T., Harris J. I. Glyceraldehyde 3-phosphate dehydrogenase: Amino acid sequence of enzyme from baker's yeast. FEBS Lett. 1972 May 1;22(2):185–189. doi: 10.1016/0014-5793(72)80040-1. [DOI] [PubMed] [Google Scholar]
- Kirschner K. Co-operative binding of nicotinamide-adenine dinucleotide to yeast glyceraldehyde-3-phosphate dehydrogenase. II. Stopped-flow studies at pH 8-5 and 40 degrees C. J Mol Biol. 1971 May 28;58(1):51–68. doi: 10.1016/0022-2836(71)90231-2. [DOI] [PubMed] [Google Scholar]
- Kirschner K., Gallego E., Schuster I., Goodall D. Co-operative binding of nicotinamide-adenine dinucleotide to yeast glyceraldehyde-3-phosphate dehydrogenase. I. Equilibrium and temperature-jump studies at pH 8-5 and 40 degrees C. J Mol Biol. 1971 May 28;58(1):29–50. doi: 10.1016/0022-2836(71)90230-0. [DOI] [PubMed] [Google Scholar]
- Kochman M., Golebiowska J., Baranowski T., Dedman J. R., Fodge D. W., Harris B. G. Hybridization of glyceraldehyde-3-phosphate dehydrogenase. FEBS Lett. 1974 Apr 15;41(1):104–107. doi: 10.1016/0014-5793(74)80964-6. [DOI] [PubMed] [Google Scholar]
- Lebherz H. G., Savage B., Abacherli E. Adenine nucleotide-mediated subunit exchange between isoenzymes of glyceraldehyde-3-phosphate dehydrogenase. Nat New Biol. 1973 Oct 31;245(148):269–271. doi: 10.1038/newbio245269a0. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Half-of-the-sites and all-of-the-sites reactivity in rabbit muscle glyceraldehyde 3-phosphate dehydrogenase. J Mol Biol. 1974 Dec 15;90(3):451–468. doi: 10.1016/0022-2836(74)90227-7. [DOI] [PubMed] [Google Scholar]
- Listowsky I., Furfine C. S., Betheil J. J., Englard S. Coenzyme-induced changes in the optical rotatory dispersion properties of glyceraldehyde 3-phosphate dehydrogenase. J Biol Chem. 1965 Nov;240(11):4253–4258. [PubMed] [Google Scholar]
- Malhotra O. P., Bernhard S. A. Activation of a covalent enzyme-substrate bond by noncovalent interaction with an effector. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2077–2081. doi: 10.1073/pnas.70.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne H. H., Hollaway M. R. The hybridization of glyceraldehyde 3-phosphate dehydrogenases from rabbit muscle and yeast. Kinetics and thermodynamics of the reaction and isolation of the hybrid. Biochem J. 1974 Dec;143(3):651–662. doi: 10.1042/bj1430651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovãdi J., Telegdi M., Batke J., Keleti T. Functional non-identity of subunits and isolation of active dimers of D-glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1971 Oct 14;22(3):430–438. doi: 10.1111/j.1432-1033.1971.tb01561.x. [DOI] [PubMed] [Google Scholar]
- PERHAM R. N., HARRIS J. I. AMINO ACID SEQUENCES AROUND THE REACTIVE CYSTEINE RESIDUES IN GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASES. J Mol Biol. 1963 Sep;7:316–320. doi: 10.1016/s0022-2836(63)80011-x. [DOI] [PubMed] [Google Scholar]
- Price N. C., Radda G. K. The binding of NAD+ to rabbit muscle glyceraldehyde-3-phosphate dehydrogenase studied by protein fluorescence quenching. Biochim Biophys Acta. 1971 Apr 14;235(1):27–31. doi: 10.1016/0005-2744(71)90029-5. [DOI] [PubMed] [Google Scholar]
- Rosemeyer M. A., Huehns E. R. On the mechanism of the dissociation of haemoglobin. J Mol Biol. 1967 Apr 28;25(2):253–273. doi: 10.1016/0022-2836(67)90141-6. [DOI] [PubMed] [Google Scholar]
- Simon I. Study of the position of NAD and its effect on the conformation of D-glyceraldehyde-3-phosphate dehydrogenase by small-angle x-ray scattering. Eur J Biochem. 1972 Oct 17;30(1):184–189. doi: 10.1111/j.1432-1033.1972.tb02085.x. [DOI] [PubMed] [Google Scholar]
- Smith G. D., Schachman H. K. Effect of D2O and nicotinamide adenine dinucleotide on the sedimentation properties and structure of glyceraldehyde phosphate dehydrogenase. Biochemistry. 1973 Sep 25;12(20):3789–3801. doi: 10.1021/bi00744a001. [DOI] [PubMed] [Google Scholar]
- Sportorno G. M., Hollaway M. R. Hybrid molecules of yeast and rabbit GPD containing native and modified subunits. Nature. 1970 May 23;226(5247):756–757. doi: 10.1038/226756a0. [DOI] [PubMed] [Google Scholar]
- Stallcup W. B., Koshland D. E., Jr Half-of-the sites reactivity and negative co-operativity: the case of yeast glyceraldehyde 3-phosphate dehydrogenase. J Mol Biol. 1973 Oct 15;80(1):41–62. doi: 10.1016/0022-2836(73)90232-5. [DOI] [PubMed] [Google Scholar]
- TUCKER D., GRISOLIA S. Inactivation of muscle triosephosphate dehydrogenase by reduced diphosphopyridine nucleotide at physiological concentrations. J Biol Chem. 1962 Apr;237:1068–1073. [PubMed] [Google Scholar]
- Trentham D. R. Aspects of the chemistry of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1968 Oct;109(4):603–612. doi: 10.1042/bj1090603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R. Rate-determining processes and the number of simultaneously active sties of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1971 Mar;122(1):71–77. doi: 10.1042/bj1220071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velick S. F., Baggott J. P., Sturtevant J. M. Thermodynamics of nicotinamide-adenine dinucleotide addition to the glyceraldehyde 3-phosphate dehydrogenases of yeast and of rabbit skeletal muscle. An equilibrium and calorimetric analysis over a range of temperatures. Biochemistry. 1971 Mar 2;10(5):779–786. doi: 10.1021/bi00781a009. [DOI] [PubMed] [Google Scholar]
- von Ellenrieder G., Kirschner K., Schuster I. The binding of oxidized and reduced nicotinamide adenine-dinucleotide to yeast glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1972 Mar 27;26(2):220–236. doi: 10.1111/j.1432-1033.1972.tb01760.x. [DOI] [PubMed] [Google Scholar]