Abstract
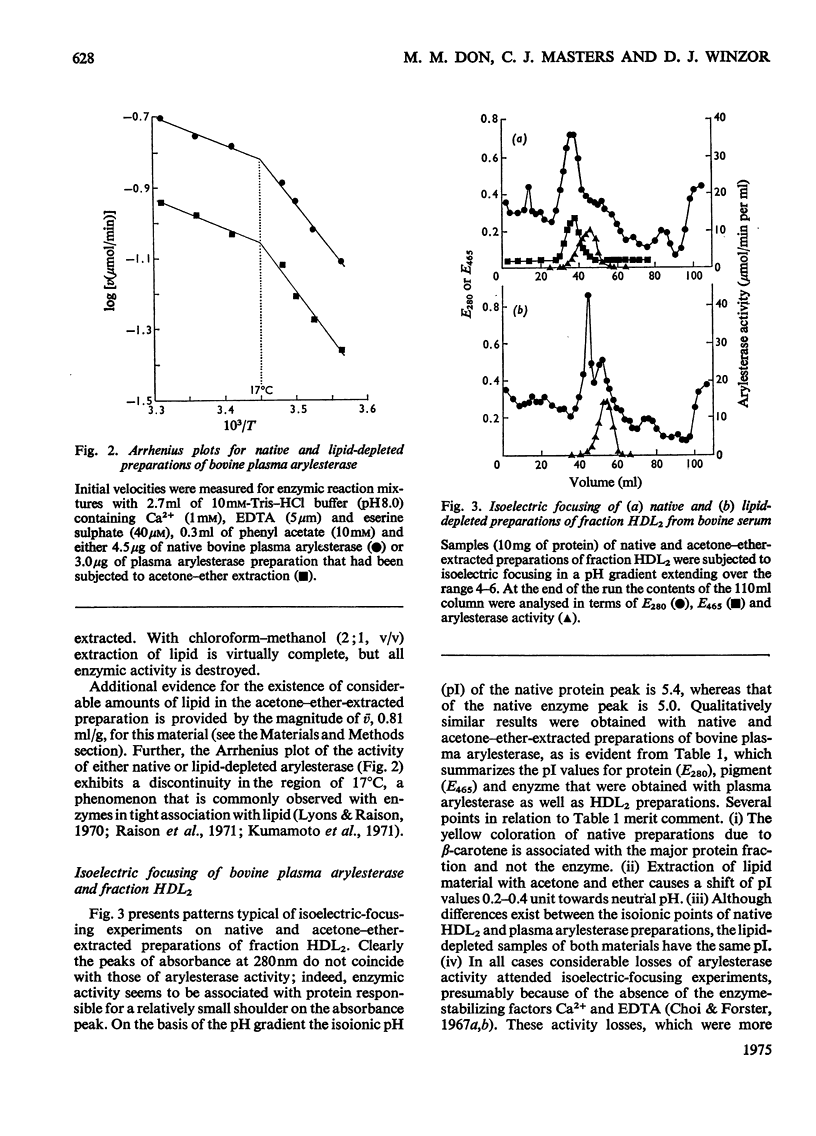
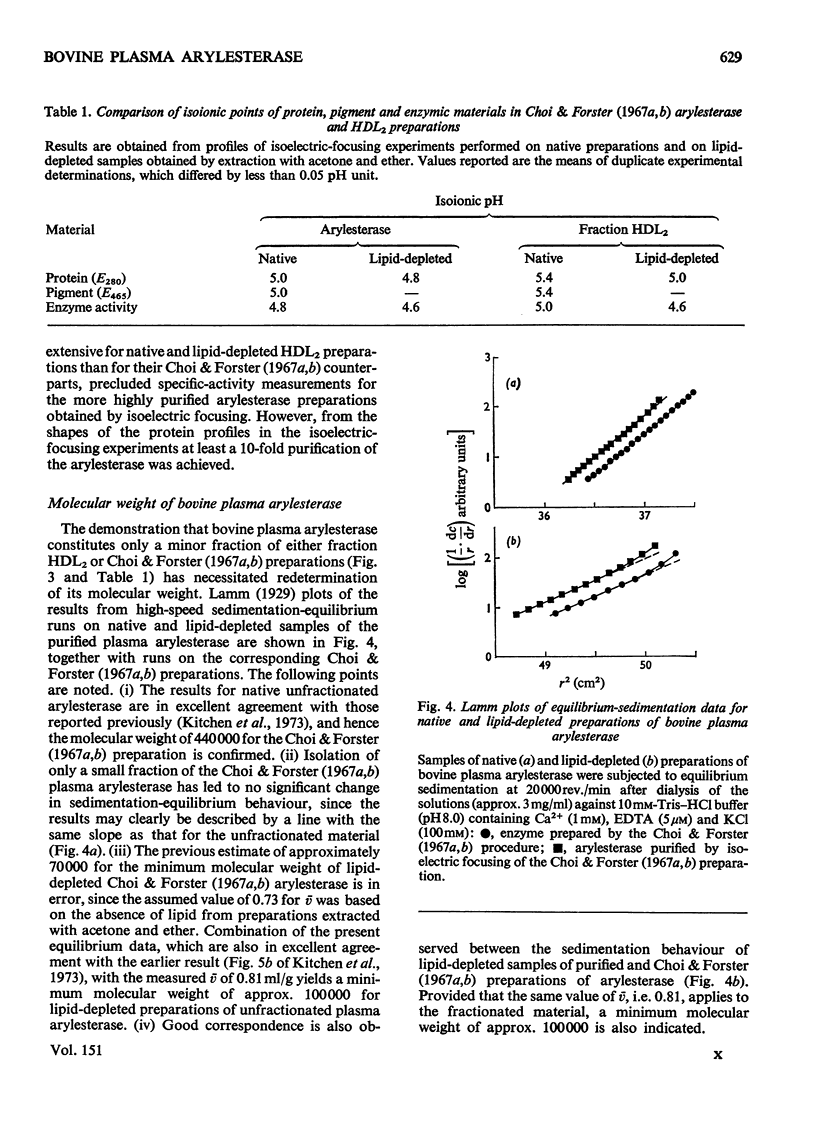
Purified preparations of bovine plasma arylesterase were obtained by isoelectric focusing of enzyme prepared by (NH4)2SO4 fractionation of plasma and chromatography on DEAE-cellulose and Sephadex G-200. Although the high-density-lipoprotein fraction (HDL2) of serum provides an alternative source of enzyme, the enzymic activity of preparations made from it is much less stable. The purified arylesterase preparation has a molecular weight of 440000 and a partial specific volume of 0.91 ml/g, properties indistinguishable from those of the less highly purified enzyme. Extraction with acetone and ether removes neutral lipids from the enzyme, but the resulting lipid-depleted preparation retains most of the phospholipid present initially. A partial specific volume of 0.81 ml/g and a minimum molecular weight of approx. 100000 were determined for the lipid-depleted preparations of arylesterase. The present results support the concept of bovine plasma arylesterase as a lipoprotein in its own right, rather than as an enzymic polypeptide that is loosely associated with the HDL2 fraction of serum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawson R. M., Clarke N. D-myoinositol 1:2-cyclic phosphate 2-phosphohydrolase. Biochem J. 1972 Mar;127(1):113–118. doi: 10.1042/bj1270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gloster J., Fletcher R. F. Quantitative analysis of serum lipids with thin-layer chromatography. Clin Chim Acta. 1966 Feb;13(2):235–240. doi: 10.1016/0009-8981(66)90298-1. [DOI] [PubMed] [Google Scholar]
- Hokin L. E., Huebner D. Radioautographic localization of the increased synthesis of phosphatidylinositol in response to pancreozymin or acetylcholine in guinea pig pancreas slices. J Cell Biol. 1967 Jun;33(3):521–530. doi: 10.1083/jcb.33.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury N., Masters C. J. Molecular weight interrelationships in the vertebrate esterases. Biochim Biophys Acta. 1970 Jan 20;200(1):58–69. doi: 10.1016/0005-2795(70)90043-7. [DOI] [PubMed] [Google Scholar]
- Kitchen B. J., Masters C. J., Winzor D. J. Effects of lipid removal on the molecular size and kinetic properties of bovine plasma arylesterase. Biochem J. 1973 Sep;135(1):93–99. doi: 10.1042/bj1350093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto J., Raison J. K., Lyons J. M. Temperature "breaks" in Arrhenius plots: a thermodynamic consequence of a phase change. J Theor Biol. 1971 Apr;31(1):47–51. doi: 10.1016/0022-5193(71)90120-2. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Thomson W. W. The influence of membranes on the temperature-induced changes in the kinetics of some respiratory enzymes of mitochondria. Arch Biochem Biophys. 1971 Jan;142(1):83–90. doi: 10.1016/0003-9861(71)90261-x. [DOI] [PubMed] [Google Scholar]
- Scanu A., Granda J. L. Effects of ultracentrifugation on the human serum high-density (1.063 less than p less than 1.21 g/ml) lipoprotein. Biochemistry. 1966 Feb;5(2):446–455. doi: 10.1021/bi00866a008. [DOI] [PubMed] [Google Scholar]
- Scott T. W., Mills S. C., Freinkel N. The mechanism of thyrotrophin action in relation to lipid metabolism in thyroid tissue. Biochem J. 1968 Sep;109(3):325–332. doi: 10.1042/bj1090325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O., Wadström T., Vesterberg K., Svensson H., Malmgren B. Studies on extracellular PROTEINS FROM Staphylococcus aureus. I. Separation and characterization of enzymes and toxins by isoelectric focusing. Biochim Biophys Acta. 1967 Apr 11;133(3):435–445. doi: 10.1016/0005-2795(67)90547-8. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]


