FIGURE 1.
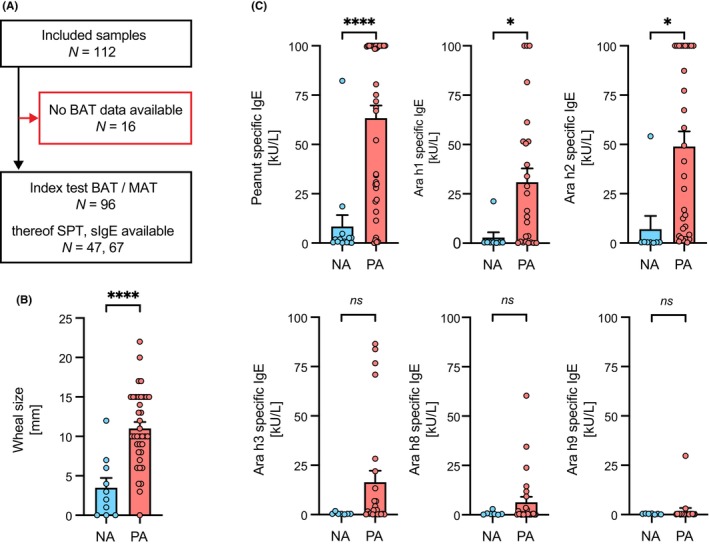
Study population with comparison of skin prick test and allergen‐specific IgE measurement results between clinically confirmed nonallergic controls and peanut allergic patients. (A) Blinded MONA study serum samples (n = 112) from the Toronto site with available clinical status were transferred to the University of Bern. Serum samples for which BAT data was recorded in MONAS (n = 96) were included in the clinical performance analysis of the Hoxb8 MAT and in direct comparison to BAT. Amongst those SPT information for 47 samples and sIgE measurements for 67 samples has been available. (B) Wheal size measurement results from skin prick test compared between nonallergic controls (NA: N = 10; blue) and peanut allergic patients (PA: N = 37; red). (C) Comparison of allergen‐specific IgE measurements by ImmunoCAP for peanut extract, Ara h1, Ara h2, Ara h3, Ara h8, and Ara h9 between nonallergic controls (NA: N = 16; blue) and peanut allergic patients (PA: N = 51; red). Data are shown as individual data points and means ± SEMs. *p < .05, ****p < .001, ns, not significant.
