Abstract
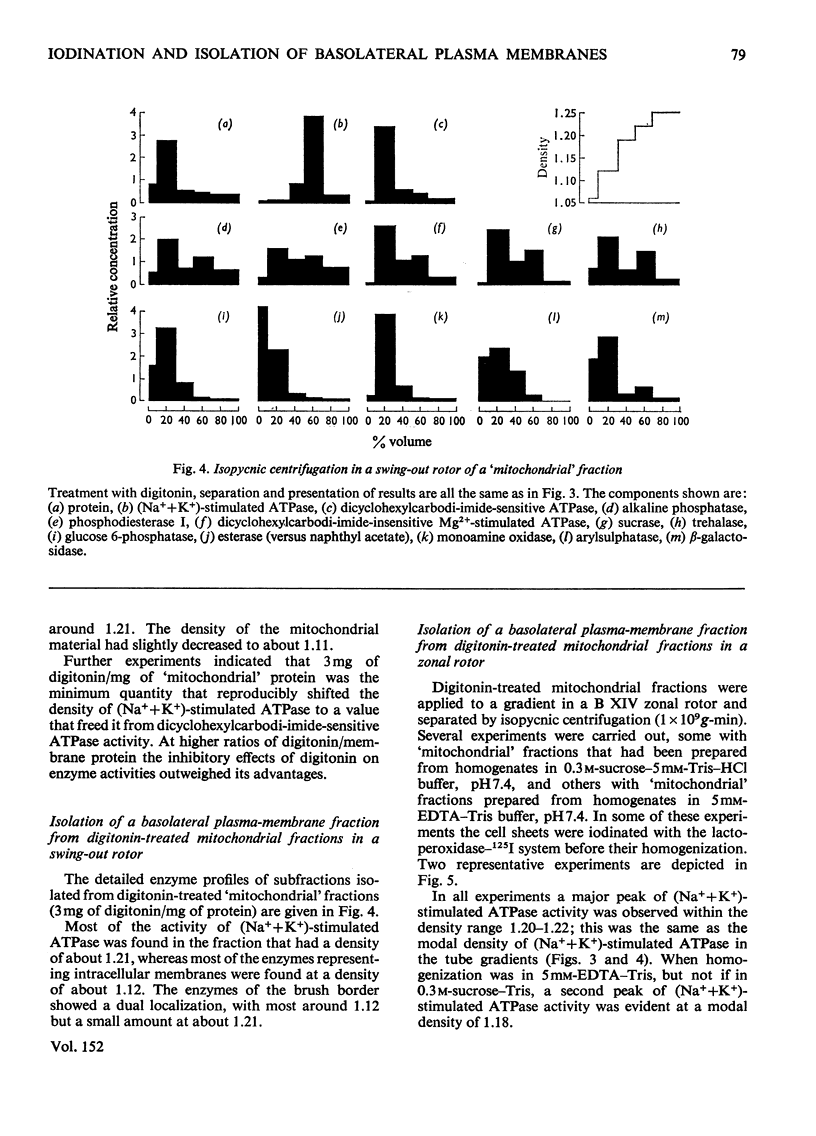
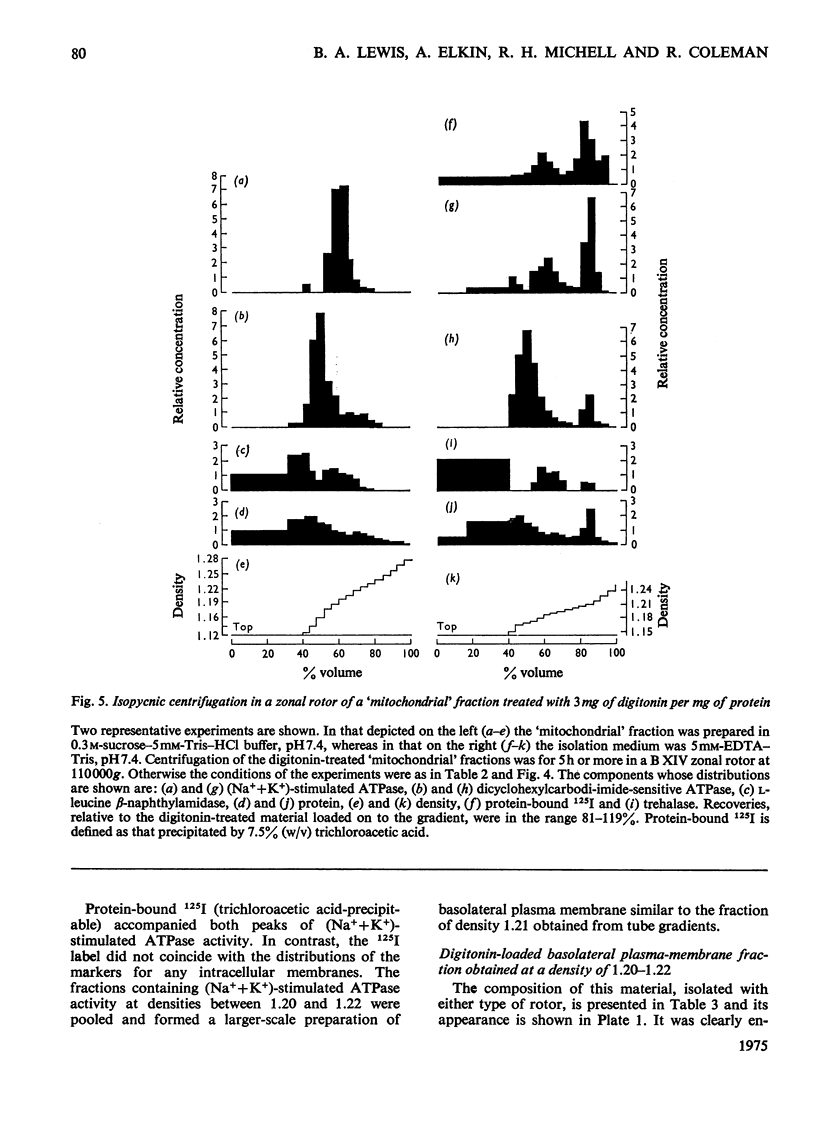
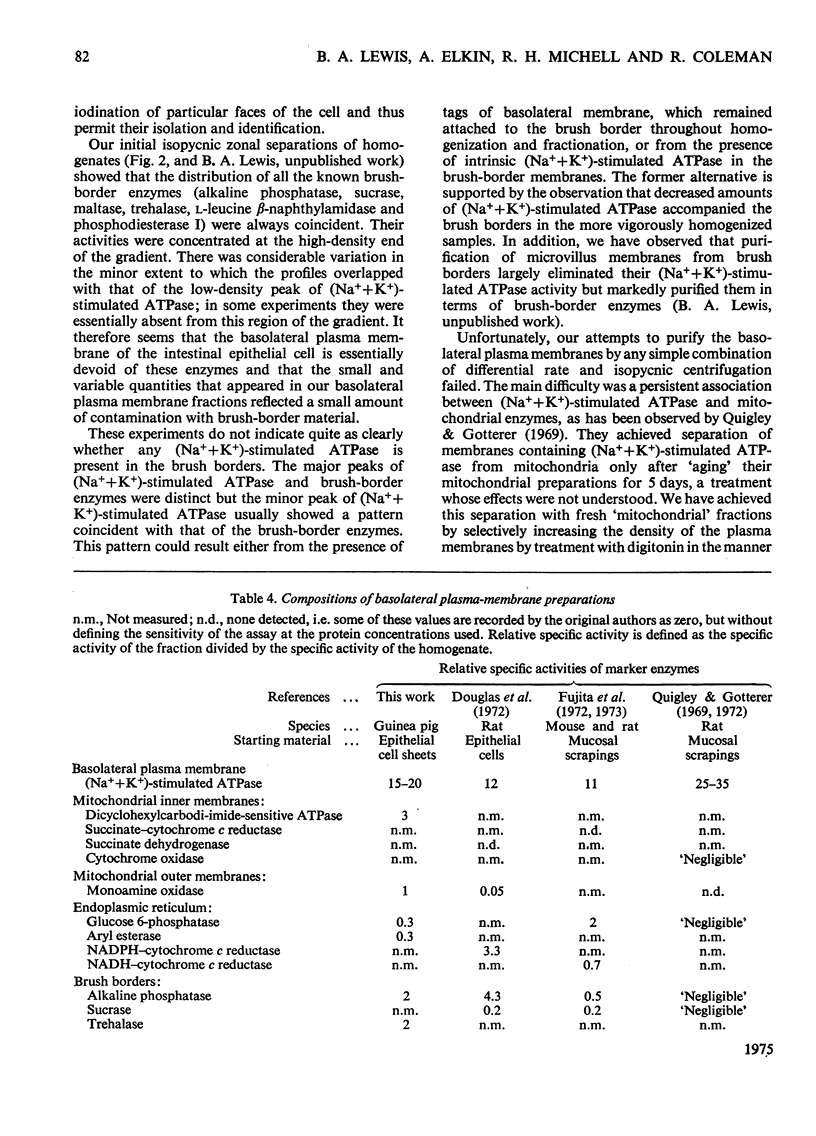
Lactoperoxidase-catalysed iodination was used to label intestinal epithelial cell sheets with 125I. The iodination was carried out under conditions that allowed little penetration of lactoperoxidase into the cells and membrane-bound 125I therefore provided an effective marker for following plasma-membrane fragments through subcellular-fractionation procedures. 2. After homogenization and isopycnic zonal centrifugation through sucrose gradients two peaks of membrane-bound 125I were detected. One coincided with brush border enzymes such as alkaline phosphatase, disaccharidases and L-leucine B-naphthylamidase, whereas the other was coincident with the major peak of (Na++K+)-stimulated ATPase (adenosine triphosphatase), which has been thought to be concentrated in the basolateral plasma membranes of these cells. Neither peak of 125I reflected the distribution of any marker for an intracellular organelle. 3. A larger proportion of the (Na++K+)-stimulated ATPase, and thus of the basolateral plasma-membrane material, was found in a crude 'mitochondrial' fraction. It was not readiily separated from mitochondria by conventional techniques of subcellular fractionation. 4. Treatment of the 'mitochondrial' fraction with digitonin increased the density of basolateral plasma membrane but had little effect on mitochondrial density. A purified preparation of digitonin-loaded basolateral plasma membranes was isolated at a density of 1.20-1.22 by isopycnic centrifugation. 5. The enzymic composition of this preparation of basolateral plasma membranes is compared with previous preparations isolated from intestinal mucosal 'scrape' materials and from isolated cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar-Costesec A., Wibo M., Thinès-Sempoux D., Beaufay H., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. IV. Biochemical, physical, and morphological modifications of microsomal components induced by digitonin, EDTA, and pyrophosphate. J Cell Biol. 1974 Sep;62(3):717–745. doi: 10.1083/jcb.62.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baginski E. S., Foà P. P., Zak B. Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin Chem. 1967 Apr;13(4):326–332. [PubMed] [Google Scholar]
- Barber A. J., Jamieson G. A. Isolation of glycopeptides from low- and high-density platelet plasma membranes. Biochemistry. 1971 Dec 7;10(25):4711–4717. doi: 10.1021/bi00801a018. [DOI] [PubMed] [Google Scholar]
- Beechey R. B., Roberton A. M., Holloway C. T., Knight I. G. The properties of dicyclohexylcarbodiimide as an inhibitor of oxidative phosphorylation. Biochemistry. 1967 Dec;6(12):3867–3879. doi: 10.1021/bi00864a033. [DOI] [PubMed] [Google Scholar]
- Brdiczka D., Pette D., Brunner G., Miller F. Kompartimentierte Verteilung von Enzymen in Rattenlebermitochondrien. Eur J Biochem. 1968 Jul;5(2):294–304. doi: 10.1111/j.1432-1033.1968.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Brightwell R., Tappel A. L. Subcellular distributions and properties of rat liver phosphodiesterases. Arch Biochem Biophys. 1968 Mar 20;124(1):325–332. doi: 10.1016/0003-9861(68)90334-2. [DOI] [PubMed] [Google Scholar]
- CRAWFORD N. An improved method for the determination of free and total cholesterol using the ferric chloride reaction. Clin Chim Acta. 1958 Jul;3(4):357–367. doi: 10.1016/0009-8981(58)90025-1. [DOI] [PubMed] [Google Scholar]
- Coleman R. Membrane-bound enzymes and membrane ultrastructure. Biochim Biophys Acta. 1973 Apr 3;300(1):1–30. doi: 10.1016/0304-4157(73)90010-5. [DOI] [PubMed] [Google Scholar]
- Connock M. J., Elkin A., Pover W. F. The preparation of brush borders from the epithelial cells of the guineapig small intestine by zonal centrifugation. Histochem J. 1971 Jan;3(1):11–22. doi: 10.1007/BF01686502. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Douglas A. P., Kerley R., Isselbacher K. J. Preparation and characterization of the lateral and basal plasma membranes of the rat intestinal epithelial cell. Biochem J. 1972 Aug;128(5):1329–1338. doi: 10.1042/bj1281329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. M., Wrigglesworth J. M., Burdett K., Pover W. F. Studies on epithelial cells isolated from guinea pig small intestine. J Cell Biol. 1971 Nov;51(21):452–464. doi: 10.1083/jcb.51.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H. Nucleotide pyrophosphatase, a sialoglycoprotein located on the hepatocyte surface. Nature. 1974 Aug 2;250(465):391–394. doi: 10.1038/250391a0. [DOI] [PubMed] [Google Scholar]
- Fujita M., Kawai K., Asano S., Nakao M. Protein components of two different regions of an intestinal epithelial cell membrane. Regional singularities. Biochim Biophys Acta. 1973 Apr 25;307(1):141–151. doi: 10.1016/0005-2736(73)90032-1. [DOI] [PubMed] [Google Scholar]
- Fujita M., Ota H., Kawai K., Matsui H., Nakao M. Differential isolation of microvillous and basolateral plasma membranes from intestinal mucosa: mutually exclusive distribution of digestive enzymes and ouabain-sensitive ATPase. Biochim Biophys Acta. 1972 Aug 9;274(2):336–347. doi: 10.1016/0005-2736(72)90181-2. [DOI] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher G., West G. R., Brindley D. N. Studies on the fractionation of mucosal homogenates from the small intestine. Biochem J. 1965 Dec;97(3):629–642. doi: 10.1042/bj0970629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Limbrick A. R., Finean J. B. X-ray diffraction and electron-microscope studies of the brush border membrane of guinea pig intestinal epithelial cells. J Cell Sci. 1970 Sep;7(2):373–386. doi: 10.1242/jcs.7.2.373. [DOI] [PubMed] [Google Scholar]
- MADDY A. H. A FLUORESCENT LABEL FOR THE OUTER COMPONENTS OF THE PLASMA MEMBRANE. Biochim Biophys Acta. 1964 Sep 25;88:390–399. doi: 10.1016/0926-6577(64)90194-9. [DOI] [PubMed] [Google Scholar]
- MILLER D., CRANE R. K. A procedure for the isolation of the epithelial brush border membrane of hamster small intestine. Anal Biochem. 1961 Jun;2:284–286. doi: 10.1016/s0003-2697(61)80014-6. [DOI] [PubMed] [Google Scholar]
- MORRISON M., HAMILTON H. B., STOTZ E. The isolation and purification of lactoperoxidase by ion exchange chromatography. J Biol Chem. 1957 Oct;228(2):767–776. [PubMed] [Google Scholar]
- MORRISON M., HULTQUIST D. E. LACTOPEROXIDASE. II. ISOLATION. J Biol Chem. 1963 Aug;238:2843–2849. [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Atwell J. L. Isolation and partial characterization of lymphocyte surface immunoglobulins. J Exp Med. 1972 Apr 1;135(4):956–971. doi: 10.1084/jem.135.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Karnovsky M. J., Karnovsky M. L. The distributions of some granule-associated enzymes in guinea-pig polymorphonuclear leucocytes. Biochem J. 1970 Jan;116(2):207–216. doi: 10.1042/bj1160207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H., Hopfer U., Kinne-Saffran E., Kinne R. Glucose transport in isolated brush-border and lateral-basal plasma-membrane vesicles from intestinal epithelial cells. Biochim Biophys Acta. 1974 Apr 29;345(2):170–179. doi: 10.1016/0005-2736(74)90256-9. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Hubbard A., Ferris B. Iodination of the human platelet membrane. Studies of the major surface glycoprotein. J Biol Chem. 1973 Apr 25;248(8):2928–2936. [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTEOUS J. W., CLARK B. THE ISOLATION AND CHARACTERIZATION OF SUBCELLULAR COMPONENTS OF THE EPITHELIAL CELLS OF RABBIT SMALL INTESTINE. Biochem J. 1965 Jul;96:159–171. doi: 10.1042/bj0960159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R. Effect of trypsin on the exposed polypeptides and glycoproteins in the human platelet membrane. Biochemistry. 1972 Nov 21;11(24):4582–4588. doi: 10.1021/bi00774a025. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. The arrangement of proteins in the human erythrocyte membrane. Biochem Biophys Res Commun. 1970 Jul 27;40(2):284–289. doi: 10.1016/0006-291x(70)91007-7. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Greenberg C. S., Glick M. C. Proteins exposed on the surface of mammalian membranes. Biochemistry. 1972 Jul 4;11(14):2616–2621. doi: 10.1021/bi00764a011. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Gotterer G. S. A comparison of the (Na + -K + )-ATPase activities found in isolated brush border and plasma membrane of the rat intestinal mucosa. Biochim Biophys Acta. 1972 Jan 17;255(1):107–113. doi: 10.1016/0005-2736(72)90012-0. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Gotterer G. S. Distribution of (Na+-K+)-stimulated ATPase activity in rat intestinal mucosa. Biochim Biophys Acta. 1969 Apr;173(3):456–468. doi: 10.1016/0005-2736(69)90010-8. [DOI] [PubMed] [Google Scholar]
- RAVIN H. A., SELIGMAN A. M. Determinant for the specificity of action of pancreatic lipase. Arch Biochem Biophys. 1953 Feb;42(2):337–354. doi: 10.1016/0003-9861(53)90363-4. [DOI] [PubMed] [Google Scholar]
- ROY A. B. The sulphatase of ox liver. I. The complex nature of the enzyme. Biochem J. 1953 Jan;53(1):12–15. doi: 10.1042/bj0530012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Schor M. T., Ng M. H. Internal localization of Micrococcus lysodeikticus membrane ATPase by iodination with 125 I. Biochim Biophys Acta. 1972 Dec 1;290(1):408–413. doi: 10.1016/0005-2736(72)90086-7. [DOI] [PubMed] [Google Scholar]
- Shephard E. H., Hübscher G. Phosphatidate biosynthesis in mitochondrial subfractions of rat liver. Biochem J. 1969 Jun;113(2):429–440. doi: 10.1042/bj1130429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetten M. R., Burnett F. F. Activation of rat-liver microsomal glucose 6-phosphatase, inorganic pyrophosphatase and inorganic pyrophosphate-glucose phosphotransferase by hydroxyl ion. Biochim Biophys Acta. 1966 Nov 15;128(2):344–350. doi: 10.1016/0926-6593(66)90181-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. G., Crawford N. The subfractionation of platelet membranes by zonal centrifugation: identification of surface membranes. FEBS Lett. 1974 May 1;41(2):317–322. doi: 10.1016/0014-5793(74)81238-x. [DOI] [PubMed] [Google Scholar]
- Thines-Sempoux D., Amar-Costesec A., Beaufay H., Berthet J. The association of cholesterol, 5'-nucleotidase, and alkaline phosphodiesterase I with a distinct group of microsomal particles. J Cell Biol. 1969 Oct;43(1):189–192. doi: 10.1083/jcb.43.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F. The dispositions of proteins in the plasma membranes of animal cells: analytical approaches using controlled peptidolysis and protein labels. Biochim Biophys Acta. 1972 Feb 14;265(1):61–83. doi: 10.1016/0304-4157(72)90019-6. [DOI] [PubMed] [Google Scholar]
- Wheeler K. P. Role of phospholipid in the intermediate steps of the sodium-plus-potassium ion-dependent adenosine triphosphatase reaction. Biochem J. 1975 Mar;146(3):729–738. doi: 10.1042/bj1460729. [DOI] [PMC free article] [PubMed] [Google Scholar]