Abstract
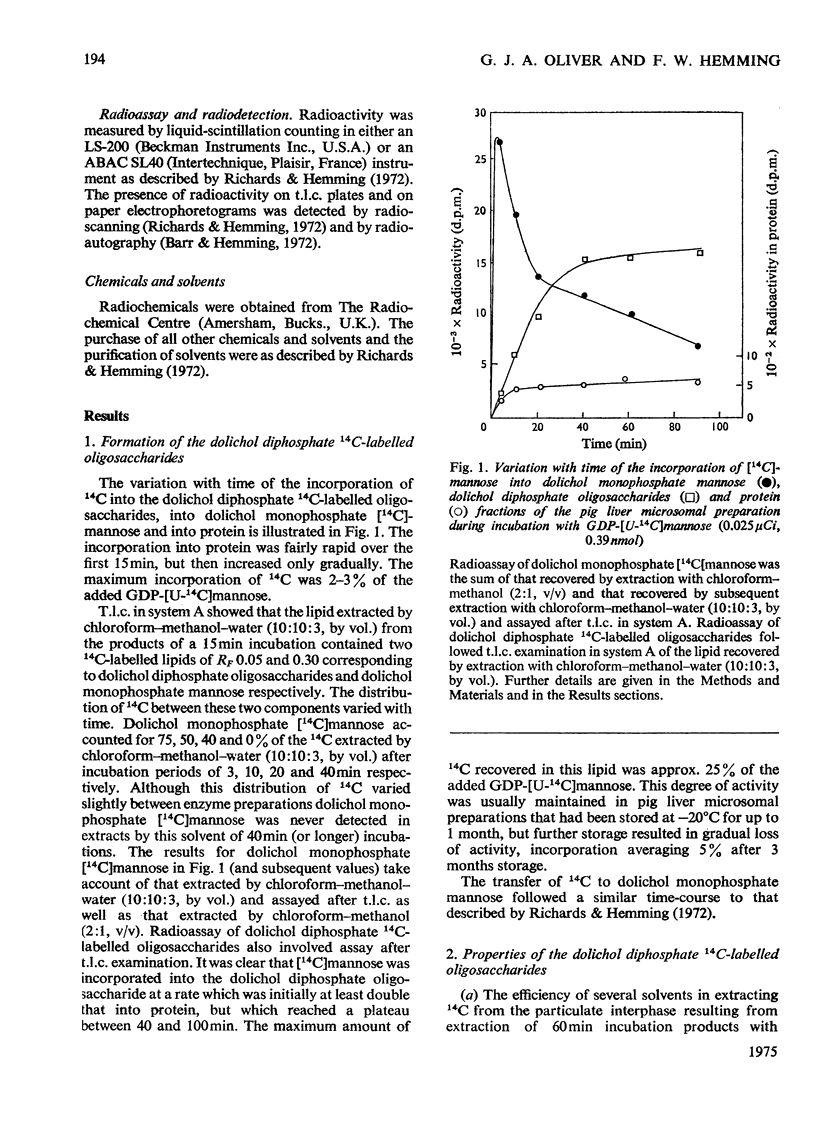
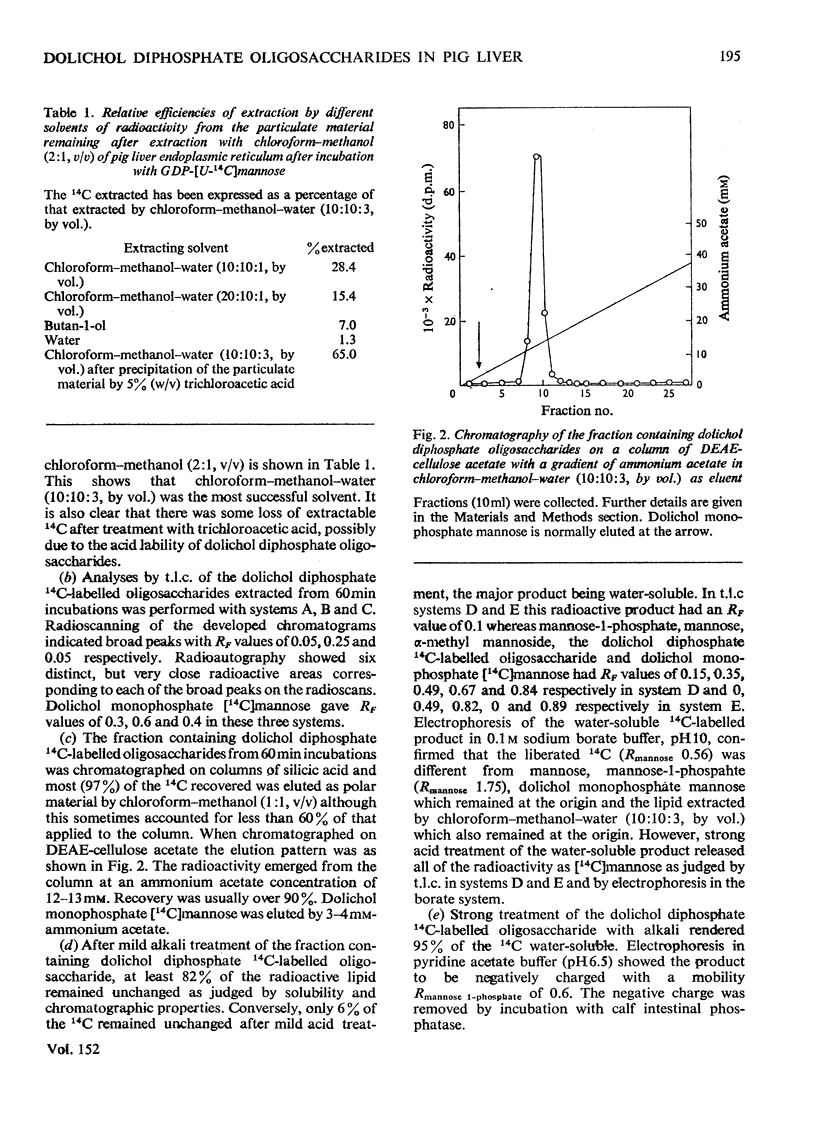
The transfer, catalysed by pig liver microsomal preparations, of mannose, from GDP-mannose, to lipid-linked oligosaccharides and the properties of the products are described. Solubility, hydrolytic and chromatographic data suggest that they are dolichol diphosphate derivatives. The presence of two N-acetyl groups in at least part of the heterogenous oligosaccharide portion was tentatively deduced. Reduction with borohydride of the oligosaccharide showed that the newly added mannose residues were not at its reducing end. Periodate oxidation suggested that 60% of these were at the non-reducing terminus and that 40% were positioned internally. T.l.c. showed the presence of seven oligosaccharide fractions with chromatographic mobilities corresponding to glucose oligomers with 7–13 residues. The molar proportions of the oligosaccharide fractions in the mixture were determined by borotritiide reduction and the number of mannose residues added to each oligosaccharide fraction during the incubation was calculated. Two of the oligosaccharide fractions had received on average one, or slightly more than one, mannose residue per chain during the incubation; four of the other fractions were each shown to be a mixture, 20–25% of which had received one mannose residue during the incubation and 75–80% of which had not been mannosylated during the incubation. This supported other evidence for the presence of endogenous lipid-linked oligosaccharides in the microsomal preparation which had been formed before the incubation in vitro. Evidence for the possibility of two pools of dolichol monophosphate mannose, one being more closely associated with mannosyl transfer to dolichol diphosphate oligosaccharides than the other, is also discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr R. M., Hemming F. W. Polyprenol phosphate as an acceptor of mannose from guanosine diphosphate mannose in Aspergillus niger. Biochem J. 1972 Mar;126(5):1203–1208. doi: 10.1042/bj1261203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens N. H., Carminatti H., Staneloni R. J., Leloir L. F., Cantarella A. I. Formation of lipid-bound oligosaccharides containing mannose. Their role in glycoprotein synthesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3390–3394. doi: 10.1073/pnas.70.12.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens N. H., Parodi A. J., Leloir L. F. Glucose transfer from dolichol monophosphate glucose: the product formed with endogenous microsomal acceptor. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2857–2860. doi: 10.1073/pnas.68.11.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. J., Hemming F. W. The unambiguous characterization of dolichol phosphate mannose as a product of mannosyl transferase in pig liver endoplasmic reticulum. FEBS Lett. 1973 May 1;31(3):335–338. doi: 10.1016/0014-5793(73)80135-8. [DOI] [PubMed] [Google Scholar]
- Hsu A. F., Baynes J. W., Heath E. C. The role of a dolichol-oligosaccharide as an intermediate in glycoprotein biosynthesis. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2391–2395. doi: 10.1073/pnas.71.6.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C., Werner D. A., Aminoff D. Quantitative determination of reducing sugars, oligosaccharides, and glycoproteins with (3H)borohydride. Anal Biochem. 1973 Sep;55(1):72–84. doi: 10.1016/0003-2697(73)90291-1. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Staneloni R., Cantarella A. I., Leloir L. F., Behrens N. H., Carminatti H., Levy J. A. Further studies on a glycolipid formed from dolichyl-D-glucosyl monophosphate. Carbohydr Res. 1973 Feb;26(2):393–400. doi: 10.1016/s0008-6215(00)84527-9. [DOI] [PubMed] [Google Scholar]
- Richards J. B., Hemming F. W. The transfer of mannose from guanosine diphosphate mannose to dolichol phosphate and protein by pig liver endoplasmic reticulum. Biochem J. 1972 Nov;130(1):77–93. doi: 10.1042/bj1300077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIRO R. G. PERIODATE OXIDATION OF THE GLYCOPROTEIN FETUIN. J Biol Chem. 1964 Feb;239:567–573. [PubMed] [Google Scholar]
- Verhue W., Hers H. G. A study of the reaction catalysed by the liver branching enzyme. Biochem J. 1966 Apr;99(1):222–227. doi: 10.1042/bj0990222. [DOI] [PMC free article] [PubMed] [Google Scholar]


