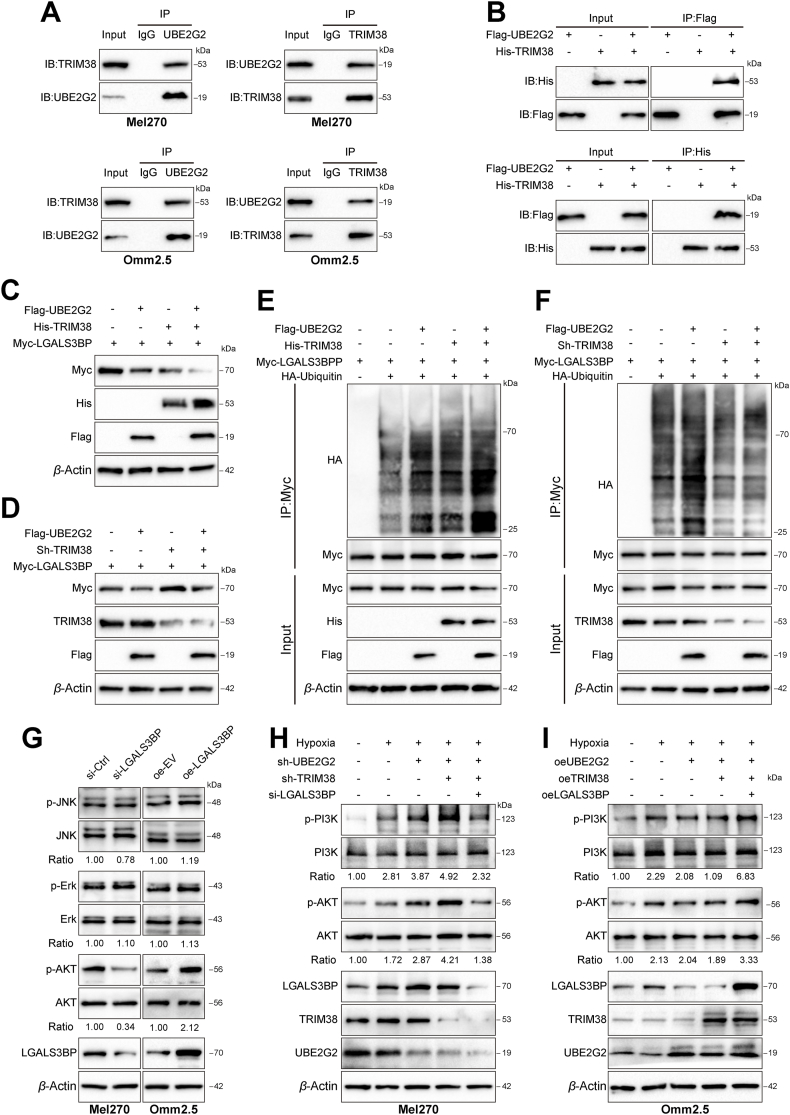
Figure 6.
TRIM38 cooperates with UBE2G2 to enhance the poly-ubiquitination and degradation of LGALS3BP and suppress the PI3K/AKT signaling pathway. (A) Co-IP and Western blotting assay in Mel270 and Omm2.5 cells tested the endogenous interaction of UBE2G2 and TRIM38. (B) HEK-293T cells were transfected with indicated plasmids, and Co-IP and Western blotting assay detected the exogenous interaction between UBE2G2 and TRIM38. (C, D) Western blotting assay was used to detect the effect of TRIM38 cooperating with UBE2G2 on regulating LGALS3BP expression. (E, F) The effect of TRIM38 cooperating with UBE2G2 on poly-ubiquitination of RPS3. HEK-293T cells expressing the indicated plasmids were treated with MG132 (10 μmol/L) for 6 h. Cell lysates were immunoprecipitated with an anti-Myc antibody, followed by immunoblotting against indicated antibodies. (G) After transfected with knockdown or ectopic LGALS3BP, the protein levels of p-PI3K, PI3K, p-Erk, Erk, p-AKT, AKT, LGALS3BP and β-actin in UM cells were measured by Western blot analysis. (H, I) After transfected with knockdown (H) or ectopic (I) lentiviruses of UBE2G2, TRIM38 and LGALS3BP, the protein levels of p-PI3K, PI3K, p-AKT and AKT in UM cells were measured by Western blot analysis. All data are presented as the mean ± SD of three independent experiments.