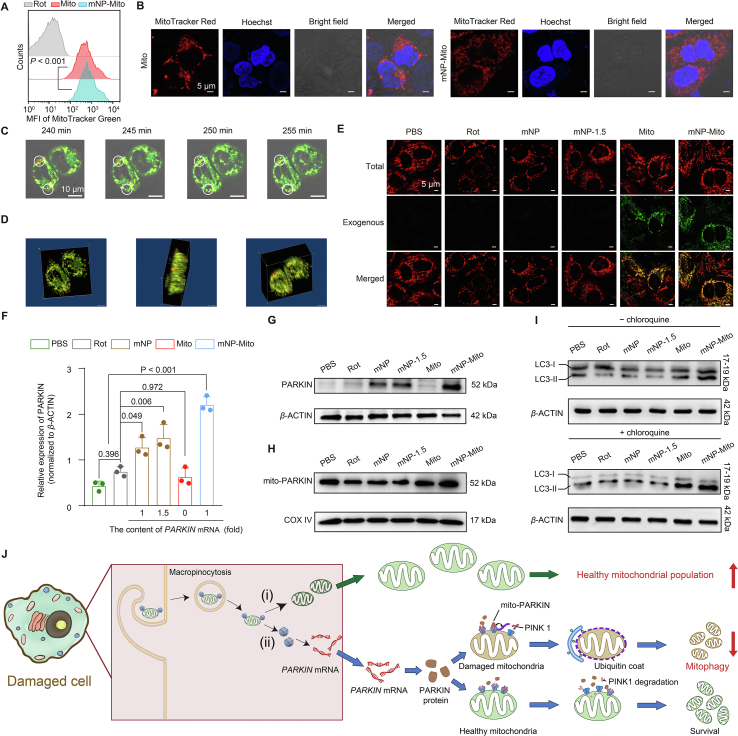
Figure 3.
Delivery of healthy mitochondria and mitophagy of dysfunctional mitochondria mediated by mNP-Mito treatment. (A, B) Delivery of exogenous healthy mitochondria detected by flow cytometry (BD Biosciences) (A) and CLSM (Carl Zeiss) (B) (24 h); (C) Internalization of MitoTracker Red labeled exogenous mitochondria in mNP-Mito investigated by live cell imaging, red: mNP-Mito, green: mitochondria within recipient cell; (D) 3D images of mNP-Mito treatment after 4 h; (E) The total mitochondria and exogenous mitochondria were respectively stained by MitoTracker Red and Green probes; (F, G) Analysis of PARKIN protein; (H) Analysis of mitochondrial PARKIN protein; (I) The expression of LC3B-II and LC3B-I proteins without (−) or with (+) chloroquine; (J) Schematic illustration of mNP-Mito treatment in the Rot-induced cell model, (i) the supplement of healthy exogenous mitochondria, (ii) the elimination of damaged mitochondria. Data are mean ± SD (n = 3 biologically independent samples in (A), (F). Statistical significance was determined using one-way ANOVA with Games–Howell test (A) and Tukey's honest significant difference (HSD) post hoc test (F).