Abstract
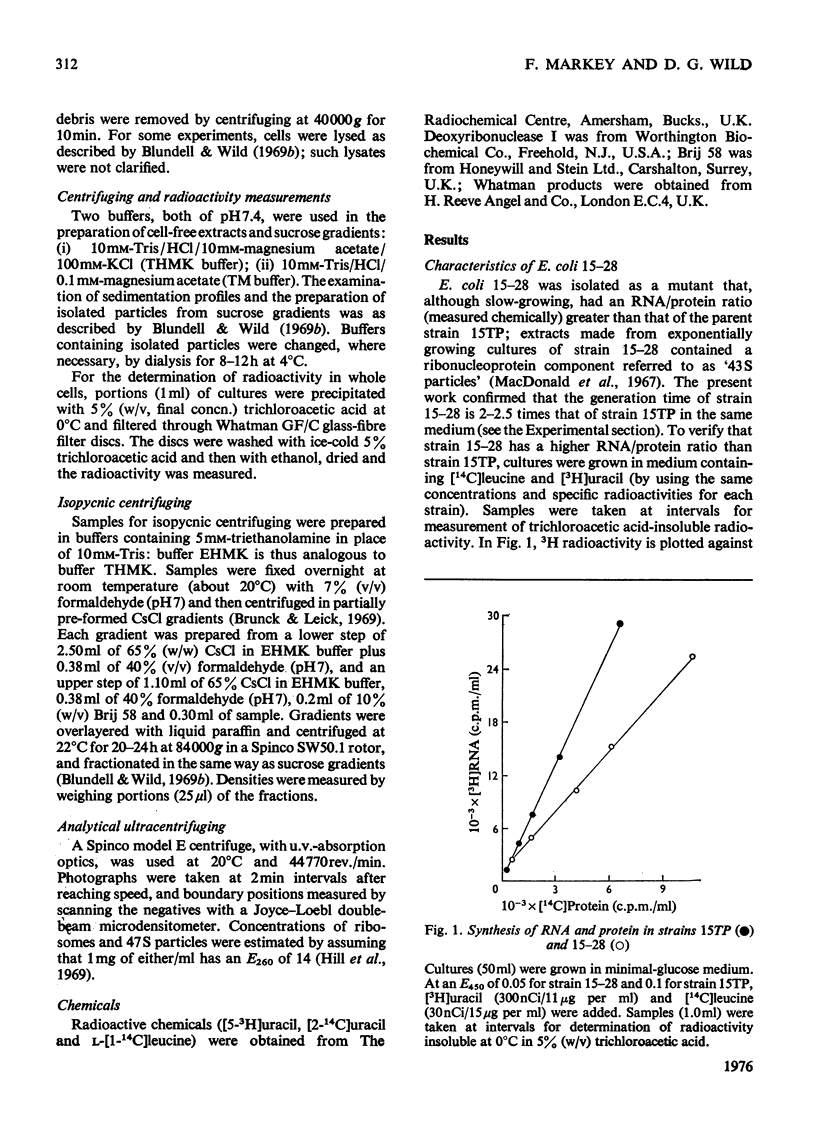
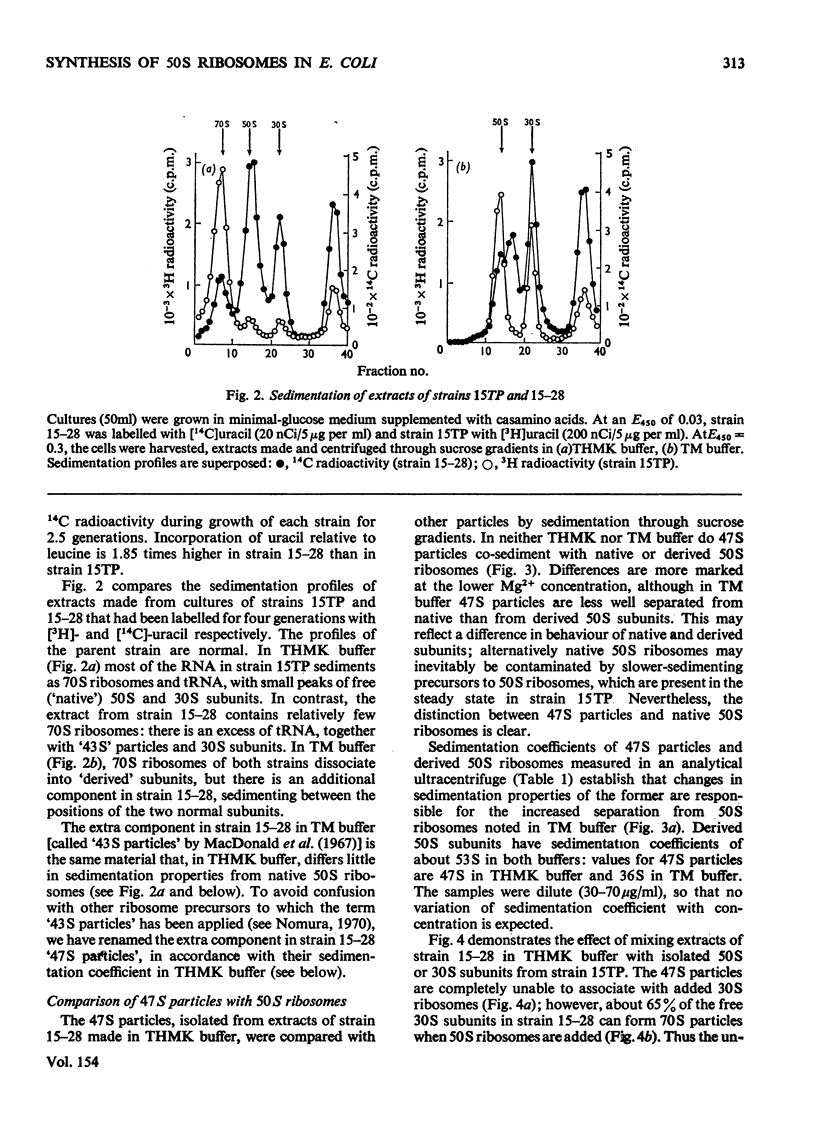
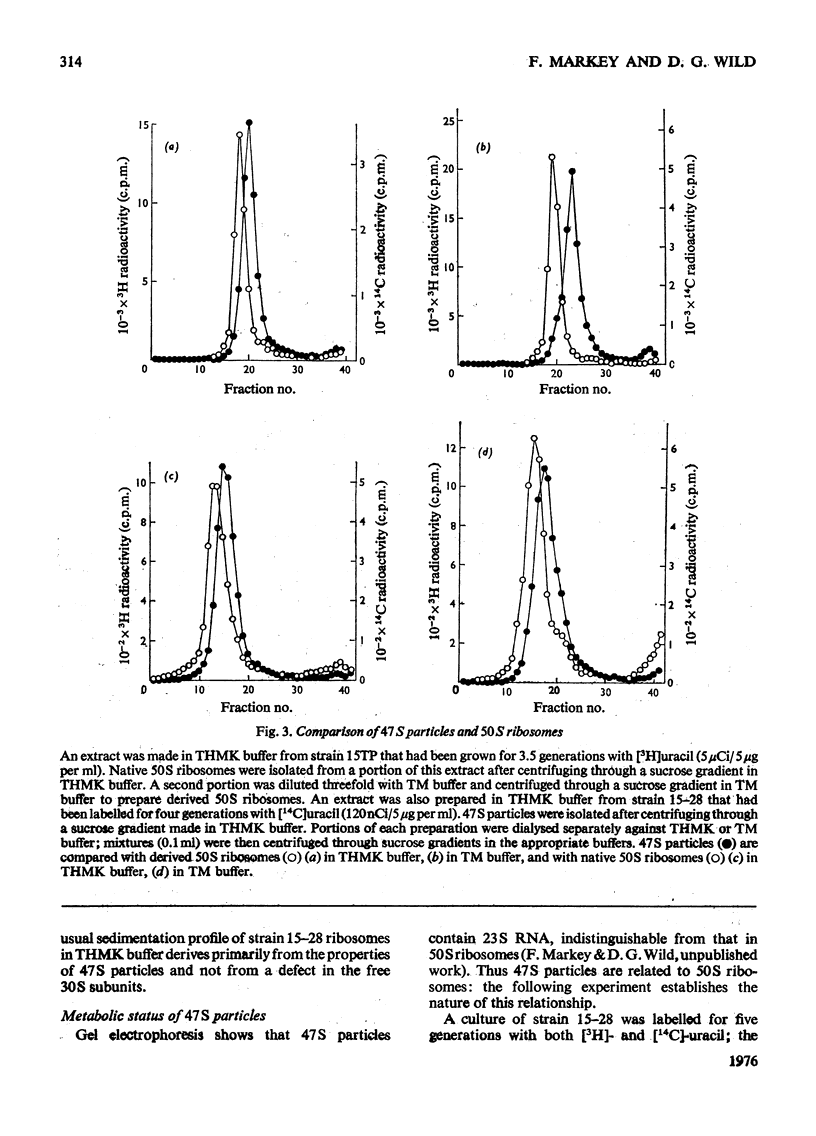
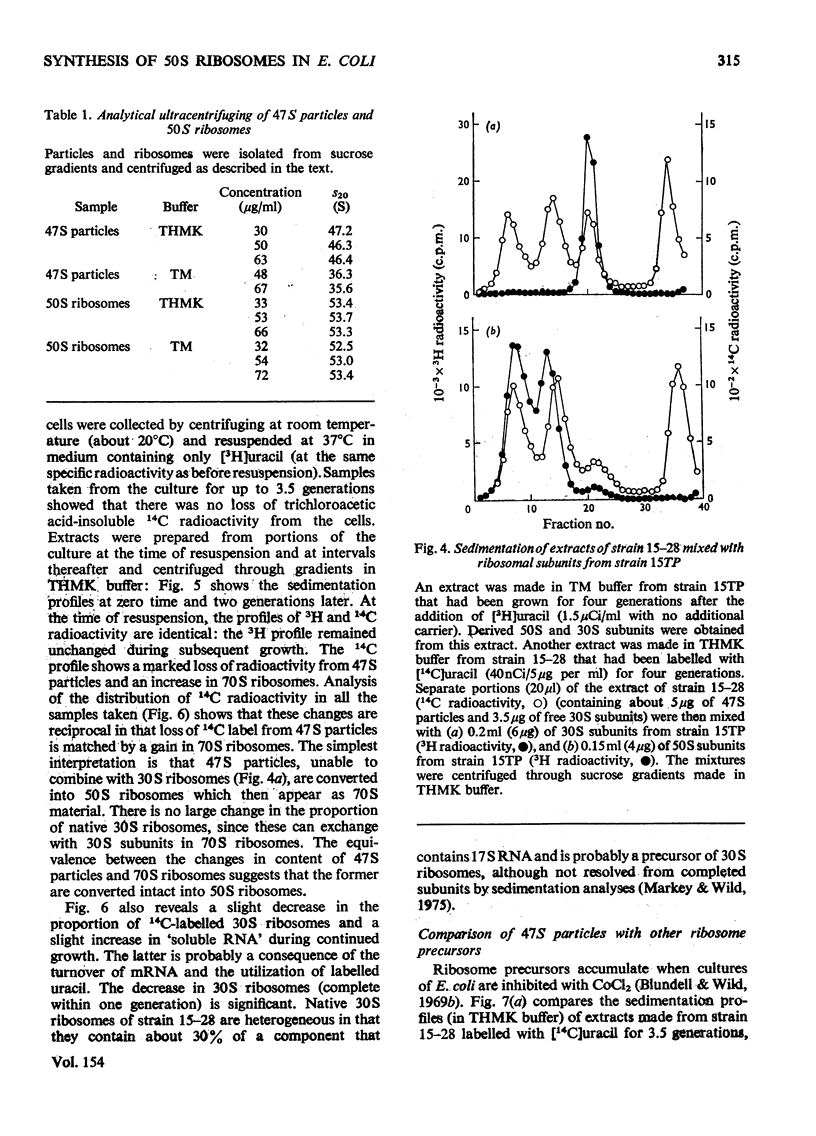
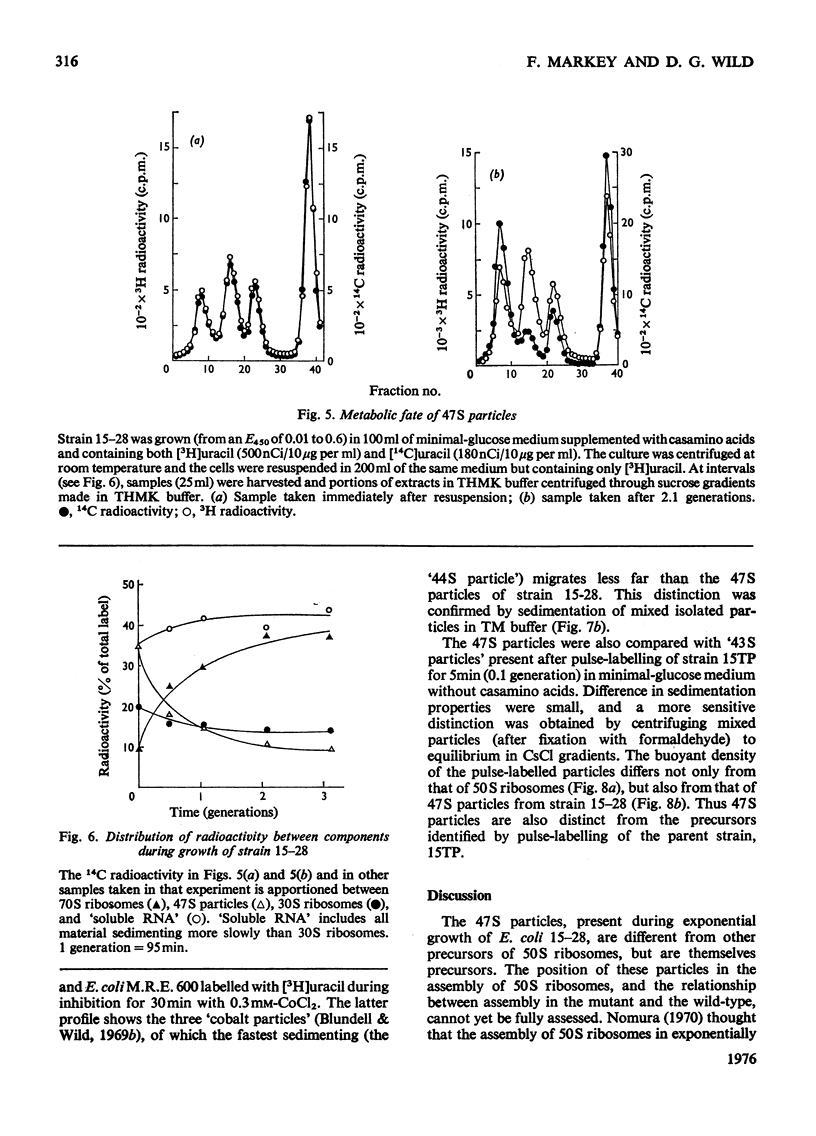
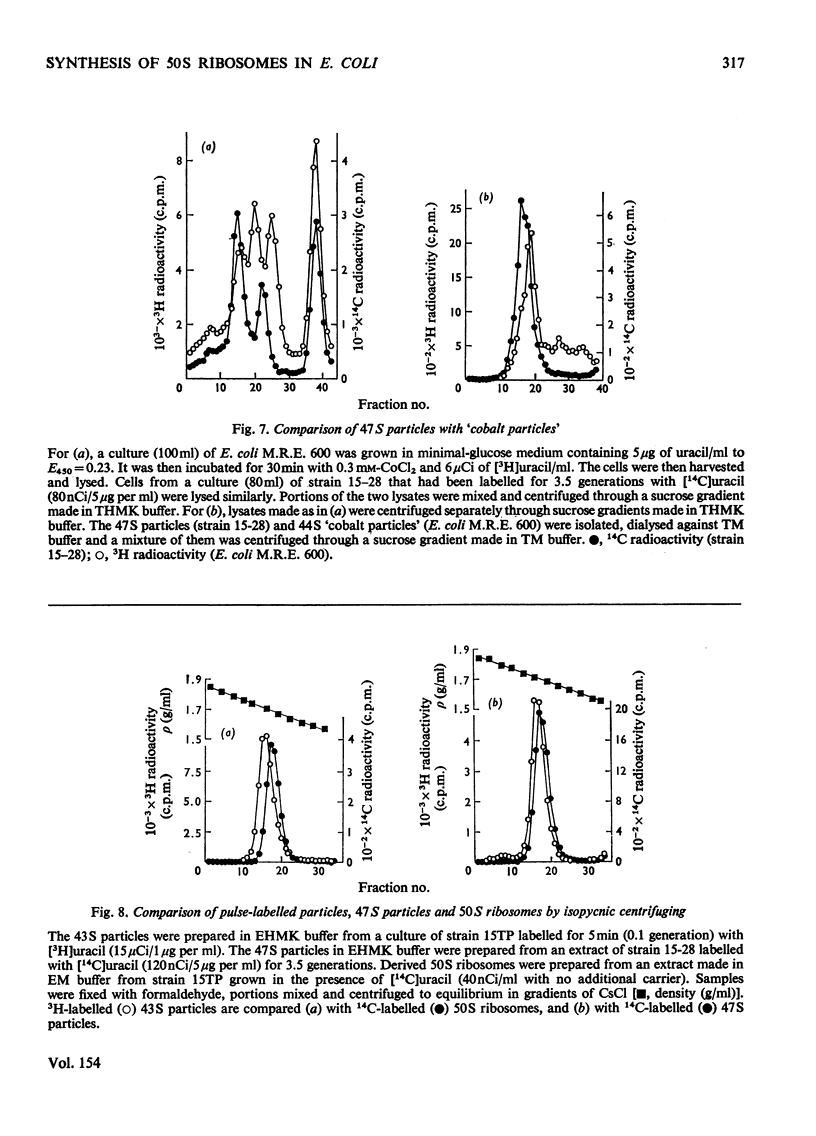
Escherichia coli strain 15-28 is a mutant with a defect in ribosome synthesis that leads to the accumulation of large amounts of ribonucleoprotein ("47S") particles during exponential growth. These particles are precursors to 50S ribosomes, but are distinct from precursors detected by pulse-labelling of the parent strain and also from ribosome precursors that accumulate during inhibition of growth by CoC12. Either ribosome assembly in the mutant differs from that in the wild-type strain, or 47S particles represent a hitherto unstudied stage in the synthesis of 50S ribosomes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blundell M. R., Wild D. G. Inhibition of bacterial growth by metal salts. A survey of effects on the synthesis of ribonucleic acid and protein. Biochem J. 1969 Nov;115(2):207–212. doi: 10.1042/bj1150207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell M. R., Wild D. G. Inhibition of bacterial growth by metal salts. The accumulation of ribonucleic acid during inhibition of Escherichia coli by cobalt chloride. Biochem J. 1969 Nov;115(2):213–223. doi: 10.1042/bj1150213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Buckel P., Ruffler D., Piepersberg W., Böck A. RNA overproducing revertants of an alanyl-tRNA synthetase mutant of Escherichia coli. Mol Gen Genet. 1972;119(4):323–335. doi: 10.1007/BF00272090. [DOI] [PubMed] [Google Scholar]
- Fiil N. P., von Meyenburg K., Friesen J. D. Accumulation and turnover of guanosine tetraphosphate in Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):769–783. doi: 10.1016/s0022-2836(72)80037-8. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Hill W. E., Rossetti G. P., Van Holde K. E. Physical studies of ribosomes from Escherichia coli. J Mol Biol. 1969 Sep 14;44(2):263–277. doi: 10.1016/0022-2836(69)90174-0. [DOI] [PubMed] [Google Scholar]
- Lewandowski L. J., Brownstein B. L. Characterization of a 43 s ribonucleoprotein component of a mutant of Escherichia coli. J Mol Biol. 1969 Apr;41(2):277–290. doi: 10.1016/0022-2836(69)90392-1. [DOI] [PubMed] [Google Scholar]
- Lindahl L. Intermediates and time kinetics of the in vivo assembly of Escherichia coli ribosomes. J Mol Biol. 1975 Feb 15;92(1):15–37. doi: 10.1016/0022-2836(75)90089-3. [DOI] [PubMed] [Google Scholar]
- López-Revilla R., Bastarrachea F. A slow-growing, streptomycin resistant mutant of Escherichia coli affected in protein synthesis and ribosomal assembly. Mol Gen Genet. 1971;113(2):99–113. doi: 10.1007/BF00333184. [DOI] [PubMed] [Google Scholar]
- Markey F., Wild D. G. A 30 S precursor of 30 S ribosomes in a mutant of Escherichia coli. Biochem J. 1975 Nov;151(2):463–465. doi: 10.1042/bj1510463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashimoto H., Held W., Kaltschmidt E., Nomura M. Structure and function of bacterial ribosomes. XII. Accumulation of 21 s particles by some cold-sensitive mutants of Escherichia coli. J Mol Biol. 1971 Nov 28;62(1):121–138. doi: 10.1016/0022-2836(71)90135-5. [DOI] [PubMed] [Google Scholar]
- Nomura M. Bacterial ribosome. Bacteriol Rev. 1970 Sep;34(3):228–277. doi: 10.1128/br.34.3.228-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S., Otaka E., Itoh T., Fukui T. Biosynthesis of 50 s ribosomal subunit in Escherichia coli. J Mol Biol. 1969 Mar 28;40(3):321–351. doi: 10.1016/0022-2836(69)90158-2. [DOI] [PubMed] [Google Scholar]
- Turnock G. A genetic analysis of a mutant of Escherichia coli with a defect in the assembly of ribosomes. Mol Gen Genet. 1969 Aug 15;104(4):295–312. doi: 10.1007/BF00334229. [DOI] [PubMed] [Google Scholar]


