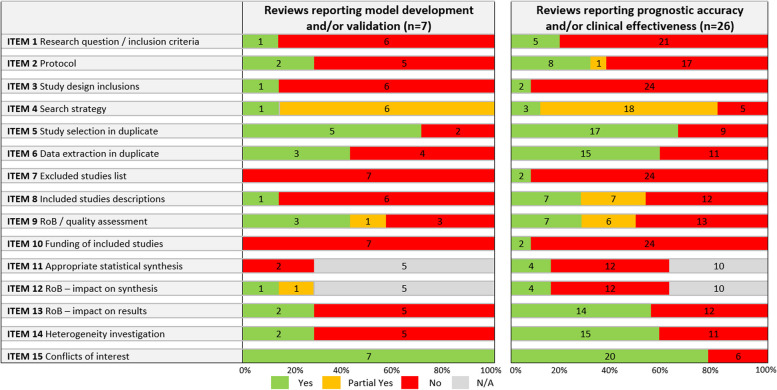
Table 2.
Summary of AMSTAR-2 assessment results
AMSTAR A MeaSurement Tool to Assess systematic Reviews, Item 1 adequate research question/inclusion criteria?, Item 2 protocol and justifications for deviations?, Item 3 reasons for study design inclusions?, Item 4 comprehensive search strategy?, Item 5 study selection in duplicate?, Item 6 data extraction in duplicate?; Item 7 excluded studies list (with justifications)?, Item 8 included studies description adequate?, Item 9 assessment of RoB/quality satisfactory?, Item 10 studies’ sources of funding reported?, Item 11 appropriate statistical synthesis method?, Item 12 assessment of impact of RoB on synthesised results?, Item 13 assessment of impact of RoB on review results?, Item 14 discussion/investigation of heterogeneity?, Item 15 conflicts of interest reported?, N/A not applicable, RoB risk of bias. Further details on AMSTAR items are given in Appendix 4, and results per review are given in Appendix 5. Note that where AMSTAR-2 assessment was applied to overlapping reviews (n = 4) for prognostic accuracy and clinical effectiveness separately, and resulted in differing judgements for each review question, the judgements for the prognostic accuracy review question are displayed here for simplicity