Figure 1.
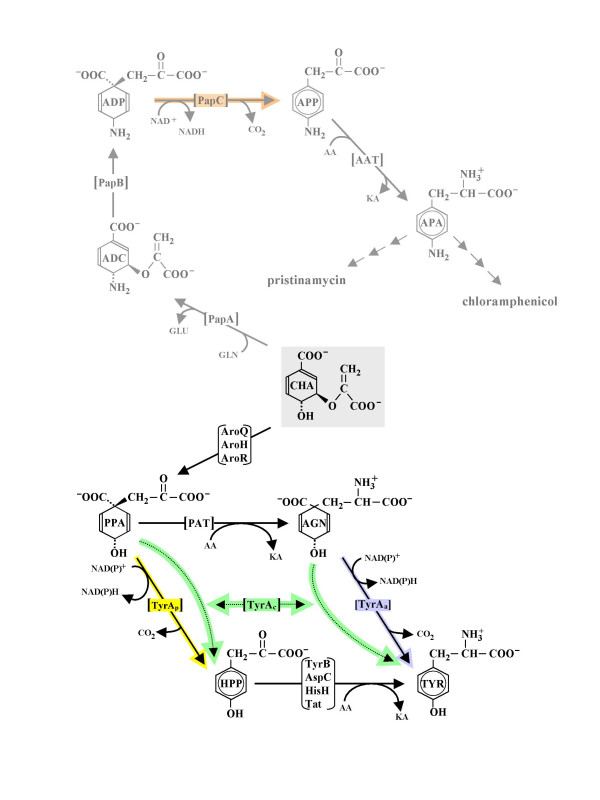
Composite of alternative biochemical routes from chorismate (CHA) to L-tyrosine (TYR) in nature. An antibiotic synthesis branch from CHA is also shown (dimmed). Here the intermediates shown to intervene between chorismate and pristinamycin or chloramphenicol are p-aminochorismate (ADC), p-aminoprephenate (ADP), p-aminophenylpyruvate (APP), and p-aminophenylalanine (APA). PPA may be transaminated by prephenate aminotransferase (PAT) to yield L-arogenate (AGN). The four TyrA homologs and the reactions they catalyze are colored differently. Arogenate dehydrogenase (TyrAa) converts AGN to TYR. Alternatively, prephenate dehydrogenase (TyrAp) converts PPA to 4-hydroxyphenylpyruvate (HPP) which is then transaminated to TYR via an homolog of TyrB, AspC, HisH, or Tat [49]. A broad-specificity cyclohexadienyl dehydrogenase (TyrAc) is competent to catalyze either the TyrAa or the TyrAp reaction. PapC converts the 4-amino analog of PPA to the 4-amino analog of HPP. AroQ, AroH, and AroR are distinct homologs known to exist in nature for performance of the chorismate mutase reaction. Other abbreviations: AA, amino acid donor, KA, keto-acid accepter.
