Abstract
While the skin is a common target organ for sarcoidosis, cutaneous granulomatous vasculitis is rare among patients with sarcoidosis. Due to the lack of detailed studies on cutaneous sarcoid vasculitis, both dermatologists and pathologists remain unfamiliar with this rare but important vasculitic disorder. We clinicopathologically evaluated eight cases with biopsy-proven cutaneous vasculitis and cutaneous sarcoidosis and analyzed morphologic changes in the process of vasculitis for both small vessels and muscular vessels in detail. The various skin lesions ranged from papulonodular erythema, annular erythema, maculopapular erythema, livedo reticularis-like eruptions, erythema nodosum-like lesions, subcutaneous nodules to ulcerative lesions. The extremities were the most frequently affected sites. Bilateral hilar lymphadenopathy with pulmonary sarcoidosis was the most common extracutaneous comorbidity. Skin-limited sarcoidosis was identified in 3 cases. All cases demonstrated a common histopathologic feature with sarcoid granulomas impinging on the target vessels with resultant vessel destruction. Perivascular infiltration of sarcoid granulomas resulted in compression and destruction of small vessels. In muscular arteries and veins, sarcoid granulomas closely attached to the muscular vessel wall, infiltrated the muscular layers and either occupied or penetrated the vessel walls, eventually invading the vascular lumen and replacing the entire muscular layers. The intimal infiltration of sarcoid granulomas resulted in a marked luminal narrowing. The scarcity of reports on cutaneous sarcoid vasculitis may be due to the overlooking or misinterpretation of vascular destruction caused by sarcoid granuloma infiltration as a feature of sarcoid granuloma masses.
Key Words: granulomatous vasculitis, sarcoidosis, sarcoid granulomas, skin
The skin is one of the organs most commonly affected by sarcoidosis after the lungs and intrathoracic lymph nodes, with skin lesions potentially occurring in one third of patients with sarcoidosis.1–3 However, cutaneous sarcoid vasculitis is rare, with only a few scattered case reports available,4–11 and to the best of our knowledge, no studies have thoroughly examined the clinical and histopathologic findings of the cutaneous vasculitis in sarcoidosis patients. As a result, most dermatologists and pathologists remain unfamiliar with this rare but important vasculitic disorder. In the present study, we conducted a detailed analysis of the morphologic changes in the process of cutaneous sarcoid vasculitis in addition to clinical evaluation and explored potential pitfalls leading to misdiagnosis or overlooking of this disease, which could improve recognition of this seemingly rare condition. Despite the diverse skin manifestations, all of the cases shared the same unique histopathologic characteristics of perivascular sarcoid granulomas compressing and destroying target vessels, which is distinct from other types of cutaneous vasculitis.
MATERIALS AND METHODS
This study included 8 patients (7 females and 1 male) with skin sarcoidosis and cutaneous vasculitis confirmed by skin biopsy at 5 medical institutions over the past 10 years. The patients were diagnosed to have either systemic sarcoidosis or cutaneous sarcoidosis. Diagnosis for skin-limited sarcoidosis was based on the examinations, including HRCT scan, chest radiology images examination, sarcoid-specific serological examinations (ACE [angiotensin-converting enzyme], lysozyme, sIL-2R, calcium levels), ophthalmologic examination, ECG and peripheral nerve function test to exclude the possible findings for systemic sarcoidosis during the period of follow-up.
The histopathologic results of skin biopsies were confirmed by 2 dermatologists and a pathologist from the coauthors, who are experts in both skin pathology and cutaneous vasculitis. Clinical records and laboratory results of each patient were reviewed from each institution. Additional serial sections were performed for elastic tissue staining and CD68 immunostaining. The former was used to differentiate the involved arteries and veins and to confirm the presence of residual destroyed vessels in areas infiltrated by sarcoid granulomas, while the latter was used to identify histiocytes in the infiltrated areas.
RESULTS
Clinical Features
The patients’ ages at diagnosis ranged from 40 to 87 years, with a mean age of 64, and there was a marked female predominance (7:1). Various skin manifestations were observed, including infiltrated erythema, papulonodular erythema, maculopapular erythema, annular erythema with central regression, subcutaneous nodules, livedo reticularis-like eruptions, erythema nodosum-like eruptions, and ulcerative lesions (Fig. 1). Different types of skin manifestations were commonly coexisted in the same patient. Bilateral hilar lymphadenopathy (BHL) and pulmonary sarcoidosis were observed in 4 patients, uveitis in 2, facial palsy in 2, and peripheral neuropathy in 1. Skin-limited sarcoidosis patients with no evidence of systemic involvement during the follow-up periods up to 3 years were found in three, while multiple comorbidities were observed in the remaining 5 patients. Patients responded well to systemic glucocorticoids up to 40 mg/d and topical steroid treatments. Three patients with skin-limited sarcoidosis showed spontaneous remission during the period of follow-up. Patients’ characteristics are shown in Table 1.
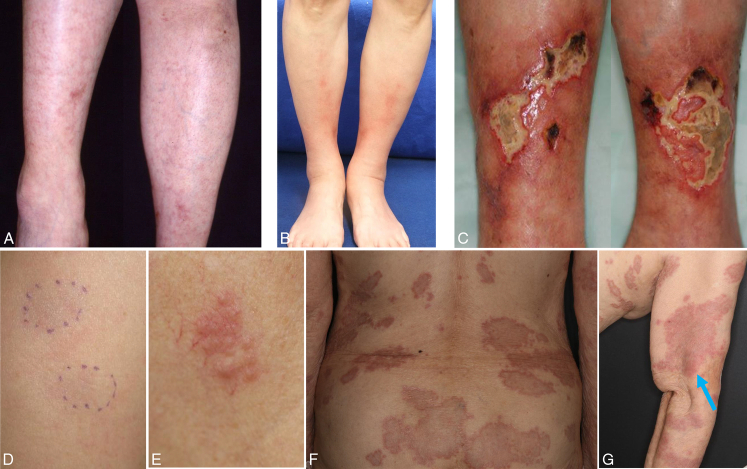
FIGURE 1.
A, Livedo reticularis-like eruption mixed with infiltrated and papular erythema on both lower legs with systemic sarcoidosis (uveitis, BHL, pulmonary sarcoidosis, facial palsy, and periphery neuropathy) in case 4. B, Lower leg erythema nodosum-like lesions in case 6. C, Lower leg ulcerations with peripheral infiltrated erythema and systemic sarcoidosis (BHL and pulmonary sarcoidosis) in case 5. D, Subcutaneous nodules on the upper arm in case 2. E, Papulonodular erythema on lt. neck with spontaneous remission in case 7. F, Widespread annular erythema with central regression over the trunk, and extremities (G) in case 8. Relatively early lesions of maculopapular erythema on the upper arm were observed (arrow) (G).
TABLE 1.
Clinical and Histopathologic Features of 8 Cases With Cutaneous Sarcoid Vasculitis
| Case/age/gender | Skin manifestation | Location | Extracutaneous sarcoidosis | Histopathologic findings | Outcome |
|---|---|---|---|---|---|
| 1/62/F | Subcutaneous nodules | Upper arm | None | Subcutaneous granulomatous phlebitis | Spontaneous regression |
| 2/66/M | Subcutaneous nodules | Lt. upper extremity | BHL elevated ACE pulmonary sarcoidosis | Subcutaneous granulomatous phlebitis with intravascular sarcoid granulomas | Unknown |
| 3/40/F | Multiple infiltrated erythema | Lower extremities | Facial palsy, myalgias elevated ACE | Lower dermal granulomatous venulitis | Unknown |
| 4/46/F | Livedo reticularis-like eruption with infiltrated erythema | Lower legs | BHL, facial palsy uveitis, pulmonary sarcoidosis Peripheral neuropathy | Mid-dermal granulomatous venulitis ~subcutaneous granulomatous venulitis and phlebitis | Improved with PSL (40 mg/d) |
| 5/81/F | Ulcerations with infiltrated erythema | Lower legs | BHL pulmonary sarcoidosis | Lower dermal granulomatous arteriolitis ~subcutaneous granulomatous arteritis | Improved with PSL (20 mg/d) |
| 6/57/F | Erythema nodosum-like eruption | Lower legs | BHL, uveitis pulmonary sarcoidosis | Lower dermal granulomatous venulitis ~subcutaneous granulomatous phlebitis | Improved with PSL (30 mg/d) |
| 7/75/F | Papulonodular erythema | Lt. neck | None | Dermo-subcutaneous junctional granulomatous arteritis with arterial muscular layers replaced by sarcoid granulomas | Spontaneous regression |
| 8/87/F | Widespread annular erythema mixed with maculopapular erythema | Extremities Trunk | None | Dermo-subcutaneous junctional granulomatous phlebitis | Spontaneous regression |
Histopathologic Features of Cutaneous Sarcoid Vasculitis
There were 2 patterns of granulomatous vasculitis affecting small vessels and muscular vessels. Features affecting both vessels were occasionally present in the same biopsy specimen.
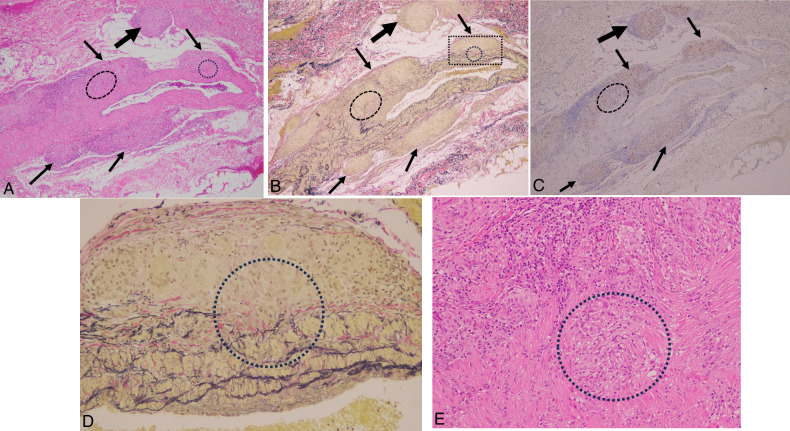
The venules from the mid-dermis to the subcutis were more commonly affected than the arterioles. The unique histopathologic features were characterized by masses of dermal or subcutaneous noncaseating epithelioid granulomas (sarcoid granulomas) impinging on and compressing or squeezing the target small vessels, with resultant destruction of these vessels (Figs. 2–4).
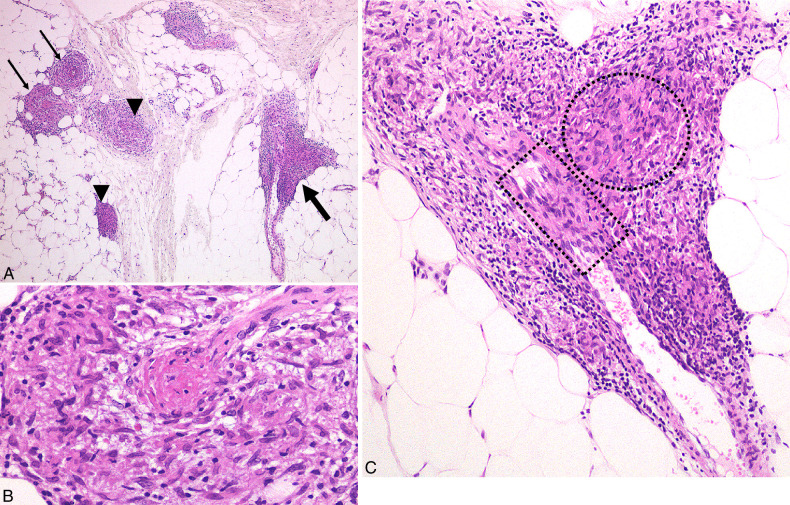
FIGURE 2.
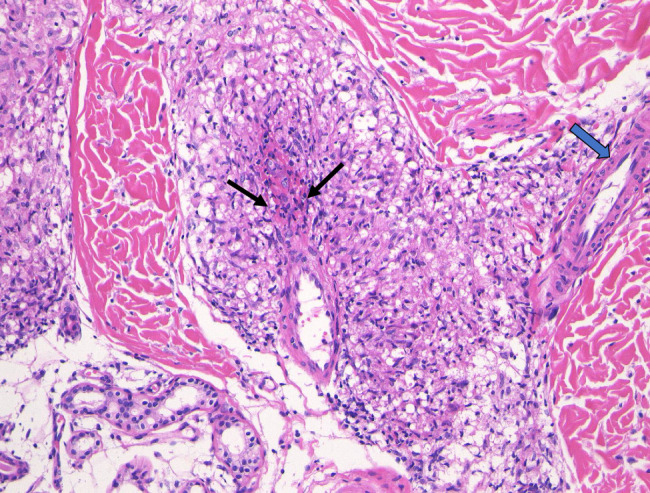
Cutaneous sarcoid venulitis in case 3 with multiple infiltrated erythema. Lower dermal venulitis with vessel wall destruction and fibrinoid necrosis (arrows) was characterized by perivenular sarcoid granulomas compressing and squeezing the target venule. Note the right-side adjacent arteriole (thick arrow) remained intact.
FIGURE 4.
Subcutaneous sarcoid venulitis and phlebitis in case 4 with livedo reticularis-like eruption. A, Sarcoid venulitis (arrows) and phlebitis (thick arrow) in association with extravascular sarcoid granulomas (arrowheads) in subcutis of the same biopsy specimen of Figure 3 showed marked perivascular infiltration of masses of sarcoid granulomas. B, Destruction of the target venous wall resulting in fibrin thrombi surrounded by sarcoid granulomas. C, Higher magnification of subcutaneous sarcoid phlebitis showed granulomatous phlebitis with masses of sarcoid granulomas (circle) impinging on the vessel wall with resultant penetration of the affected vein and sarcoid granuloma in the vascular lumen (rectangle).
Both muscular arteries and veins from the dermo-subcutaneous junction to subcutis showed sarcoid granulomas closely attached to the muscular vessel walls (Figs. 4C, 5, 6, 8–11), subsequently infiltrating the muscular and intimal layers (Figs. 4C, 5, 6, 8–10). As a result, sarcoid granulomas either penetrated the venous wall and persisted in the venous lumen (Figs. 4C, 6), or caused the entire muscular layer to be occupied (Figs. 8, 9B) or even replaced (Fig. 9B) by sarcoid granulomas, and eventually leading to narrowing of the lumen (Figs. 6, 8, 9B).
FIGURE 5.
Cutaneous sarcoid phlebitis in case 6 with erythema nodosum-like eruption. A, Early lesion of cutaneous sarcoid phlebitis Cannonball-like masses of sarcoid granulomas impinging on and compressing (arrows) the target vein resulting in extreme thinness of the affected venous wall (oval). B, Subcutaneous granulomatous phlebitis encircled by outer elastic fibers (arrowheads) showed infiltration of sarcoid granulomas (thick arrows) occupying both vessel wall and perivascular area with fibrinoid necrosis (arrow). C, Elastic fibers (arrowheads) encircled phlebitis showed infiltration of masses of sarcoid granulomas (thick arrows) in and outside the target vein with perivascular fibrinoid necrosis (arrow) (EVG staining). D. Infiltration of CD68-positive sarcoid granulomas (thick arrows) encircled and occupied the vessel wall of the target vein.
FIGURE 6.
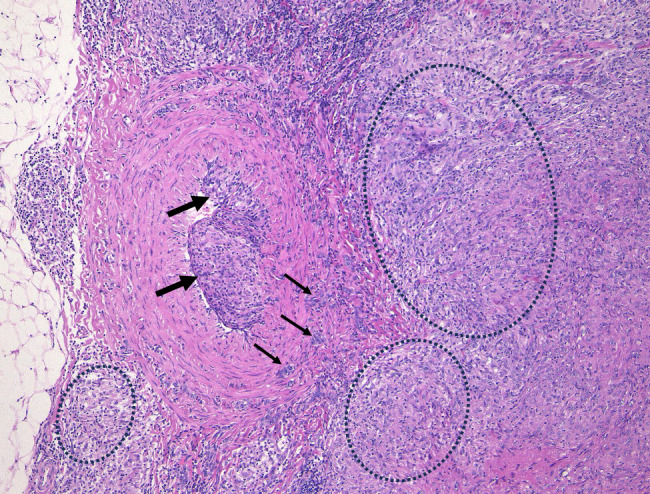
Subcutaneous sarcoid phlebitis with fully developed sarcoid granulomas in the lumen in case 2 with subcutaneous nodules. The target muscular vein was compressed and infiltrated by perivascular masses of sarcoid granuloma (ovals) with scattered sarcoid granulomas (arrows) in the muscular layers. Development of intravascular sarcoid granulomas (thick arrows) resulted in marked luminal narrowing.
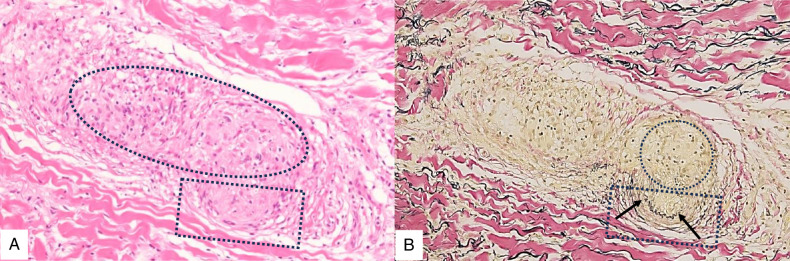
FIGURE 8.
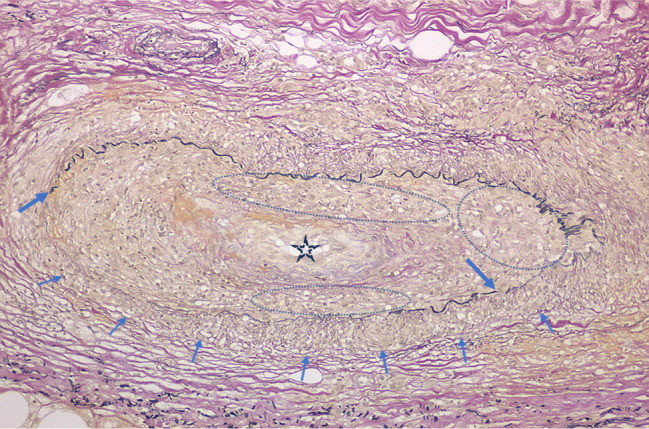
Cutaneous sarcoid arteritis of the same biopsy specimen in Figure 7. Dermo-subcutaneous junctional sarcoid arteritis with infiltration of sarcoid granulomas in the muscular layer (arrows) and intima (ovals) resulting in disruption of the internal elastic lamina (thick arrows) and intimal cellular fibrous thickening with narrowing of the lumen (star) (EVG staining).
FIGURE 11.
Comparison between cutaneous sarcoid arteritis and cutaneous arteritis. A, Disrupted external elastic lamina (arrows) in the early lesion of sarcoid arteritis was caused by perivascular sarcoid granulomas (oval) impinging on and closely attaching to the muscular vessel wall (EVG staining). B, In contrast, disrupted external elastic lamina (arrows) in the subacute lesion of cutaneous arteritis was caused by luminal inflammatory cells discharging from the portions of the disrupted internal elastic lamina (thick arrows) and spreading outward to the muscular layer and perivascular areas (oval) (EVG staining).
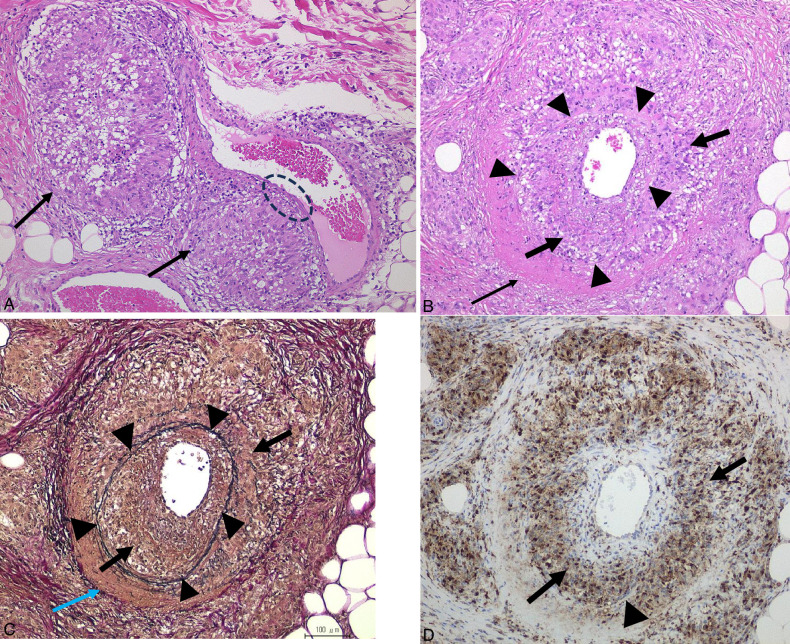
FIGURE 10.
Cutaneous sarcoid phlebitis in case 8 with widespread annular erythema. A, Perivascular infiltration of masses of sarcoid granulomas (arrows) closely attached to the vessel wall of the target vein at the dermo-subcutaneous junction with adjacent overlying naked granuloma (thick arrow). B, EVG staining indicates the affected vessel is a muscular vein with loss of elastic tissue of the left side muscular layer due to space-occupying sarcoid granuloma in the affected muscular layer (oval). C, Positive CD68 staining in the infiltrate of sarcoid granulomas (arrows) and the adjacent naked granuloma (thick arrow). D, Early stage of granulomatous phlebitis with higher magnification of the rectangle in B showed a mass of sarcoid granuloma closely attached to the venous wall with an underlying local infiltrate of sarcoid granuloma in the affected muscular layer (circle) (EVG staining). E, Higher magnification of the ovals in A–C revealed deeper infiltration of sarcoid granuloma in the muscular layer of the affected vein resulting in the replacement of the muscular layers with sarcoid granuloma (circle).
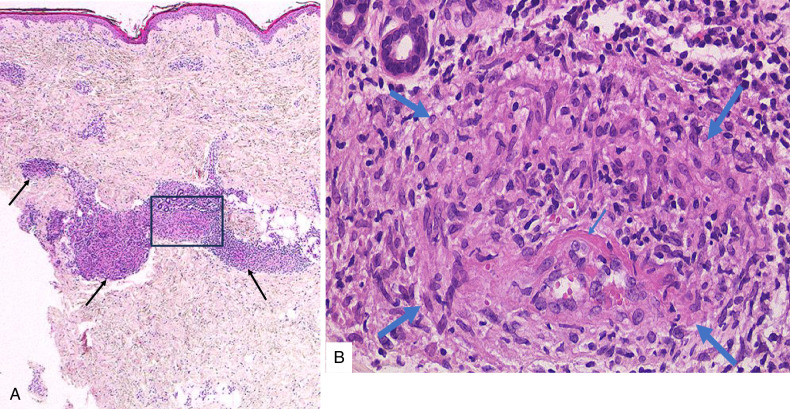
FIGURE 9.
Cutaneous sarcoid arteritis in case 7 with papulonodular erythema. A, Diffuse infiltrate of sarcoid granulomas in the dermis involving a target artery at the dermo-subcutaneous junction (arrow). B, Higher magnification of the affected artery showed a unique feature of granulomatous arteritis with the entire arterial muscular layer replaced by marked sarcoid granulomas (double circles) and intimal fibrinoid necrosis.
Features affecting both small vessels and muscular vessels were present in the same biopsy specimen (Figs. 3, 4, 7, 8).
FIGURE 3.
Cutaneous sarcoid venulitis in case 4 with livedo reticularis-like eruption. A, Lower dermal granulomatous venulitis (rectangle) in association with cluster of dermal sarcoid granulomas (arrows). B, Higher magnification of A (rectangle) showed the surrounding sarcoid granulomas (thick arrows) impinging on and compressing the target dermal venule with resultant vessel wall fibrinoid necrosis (arrow) and extravasation of red blood cells at the periphery.
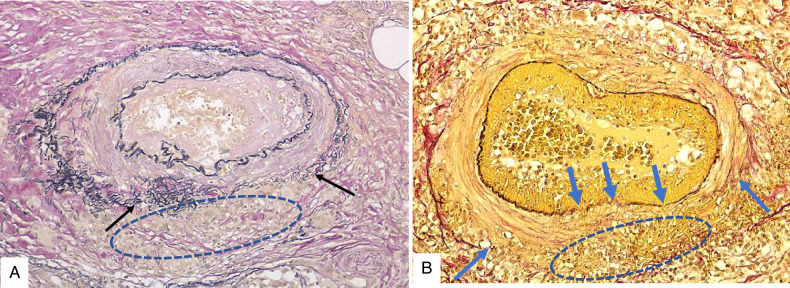
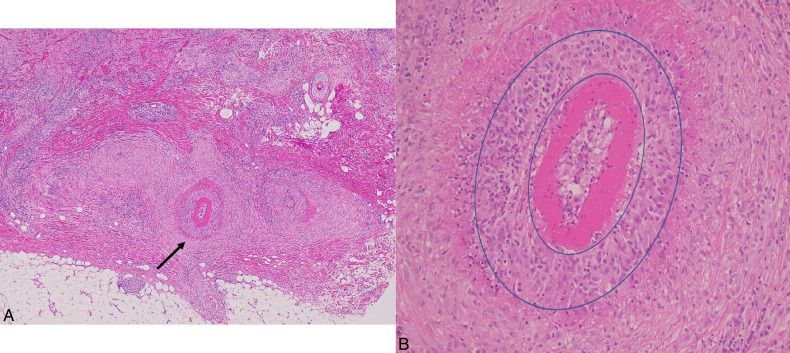
FIGURE 7.
Cutaneous sarcoid arteriolitis in case 5 with lower leg ulcerations. A dermal arteriole affected by the left upper side infiltration of masses of sarcoid granulomas (oval) resulted in compressing and replacing the upper half of the affected arteriole with sarcoid granuloma and the remaining residual lower part of the target arteriole (rectangle). B, Elastica van Gieson (EVG) staining identified loss of the upper part vessel wall replaced by a mass of sarcoid granuloma (circle) and the lower part of the remaining intact arterial wall (rectangle) with its disrupted internal elastic lamina (arrows). It should be noted that it may easily overlook the residual disrupted arteriole in the infiltrate of sarcoid granulomas in A if EVG staining were not performed to identify the existence of the residual disrupted arteriole in B.
DISCUSSION
Cutaneous vasculitis in sarcoidosis has been reported in 2 forms: granulomatous vasculitis,4–11 as seen in the present cases, and leukocytoclastic vasculitis, observed in acute cases.12–16 Leukocytoclastic vasculitis in sarcoidosis belongs to the category of immune complex-related vasculitis and presents as a superficial cutaneous vasculitis secondary to sarcoidosis. Cutaneous manifestations are characterized by palpable purpuric plaques12,13 or annular purpuric patches,14–16 and histopathologic features include characteristics of leukocytoclastic vasculitis with neutrophils in the infiltrate limited to the superficial dermis. These features are completely different from those observed in the present cases of granulomatous vasculitis, which showed involvement of deep vessels from mid-dermal to subcutis and massive sarcoid granulomas in the infiltrate. Moreover, cases of acute systemic sarcoidosis with leukocytoclastic vasculitis usually lack the histopathologic findings of sarcoid granulomas in their purpuric lesions.12–16 Sarcoid vasculitis is listed in the category of systemic vasculitis in the 2012 Revised International Chapel Hill Consensus Conference (CHCC) Nomenclature of Vasculitis17 and D-CHCC 201818 for vasculitis definition. Although the skin is one of the most commonly affected organs in sarcoidosis after the lung, cutaneous sarcoid vasculitis has rarely been reported,4–11 and to date, no detailed studies have described the morphologic process of vessel destruction in this condition.
Sarcoid vasculitis is most often found in the lungs,19–21 where the incidence of sarcoidosis is higher compared with other organs. Sarcoid vasculitis was reported to be present in 53% of patients with sarcoidosis via transbronchial biopsy.19 All levels of blood vessels in the lungs are involved, with a higher frequency of involvement in the veins than in the arteries.20,21 In the present study, almost all vascular levels, except those in the superficial dermis, were involved, and similar to prior studies, the venules from the mid-dermis to subcutis were more commonly affected than the arterioles. In our study, although there was a limited number of cases, we did not observe any instances of granulomatous vasculitis in the superficial dermis (Table 1). Sarcoid vasculitis in the lungs showed similar vessel wall destruction characterized by perivascular infiltration of sarcoid granuloma masses attaching to the vessel walls with subsequent infiltration in the target vessel walls, resulting in occupying and replacing the muscular layer by sarcoid granulomas.20,21 Similar vessel destruction caused by sarcoid granuloma infiltration has also been identified in other organs, such as the kidney.22
Cutaneous granulomatous vasculitis can also be seen in disorders such as EGPA (Churg-Strauss syndrome),23,24 nodular vasculitis, collagen diseases, lymphoproliferative disorders, inflammatory bowel diseases, autoimmune inflammatory diseases, and infection.25,26 However, these disorders were excluded in the present study due to the coexistent histopathologic features of cutaneous sarcoidosis and the unique feature of cannonball-like vessel destruction by sarcoid granulomas in the same biopsy specimens (Figs. 3–5, 9, 10).
The unique feature of vessel destruction is also distinct from most other types of cutaneous vasculitis, including leukocytoclastic vasculitis of small vessels and cutaneous arteritis of muscular vessels, which are characterized by inflammatory cells, primarily neutrophils migrating from the vessel lumen to the outer layers. A comparison between cutaneous sarcoid arteritis and cutaneous arteritis27,28 is shown in Figure 11.
The coexistence of dermal and subcutaneous sarcoidosis in patients with cutaneous sarcoid granulomatous vasculitis could account for their various skin manifestations as commonly seen in systemic or skin-limited sarcoidosis without findings of vasculitis.1–3
As cutaneous sarcoid vasculitis always coexisted with cutaneous sarcoidosis, and only one skin incisional biopsy was conducted in this study, it is hard to conclude whether other nonbiopsied skin lesions clinically similar to the biopsy-proven vasculitic lesions could have only cutaneous sarcoidosis without evidence of vasculitis or the same findings as seen in this study.
Cutaneous granulomatous vasculitis in sarcoidosis may not be as extremely rare as documented. We have previously reviewed 111 cases of skin biopsy-proven cutaneous sarcoidosis from patients with either systemic sarcoidosis or skin-limited cases in our institution (Fukushima Medical University) over the last 17 years and identified 2 cases of cutaneous sarcoid vasculitis that were not included in this study and had been reported as case report in 2 different journals,9,11 respectively. However, when we re-evaluated the biopsy specimens of these cutaneous sarcoidosis cases after that, we identified another three cases due to overlooking the presence of granulomatous vasculitis in the nodular infiltrates of sarcoid granulomas previously.
Based on our experience, this discrepancy may be due to misinterpretation or overlooking of the features of vessel destruction as part of the features of dermal or subcutaneous infiltration of sarcoid granulomas. This pitfall could be avoided by additional elastic tissue staining to confirm the existence of the remaining muscular layer with disrupted internal elastic lamina of the affected arterioles (Fig. 7B), which became unclear in the infiltrate of sarcoid granulomas in H&E staining (Fig. 7A).
Nevertheless, the 4.5% (5/111) incidence of sarcoid vasculitis among patients with cutaneous sarcoidosis in our institution indicated the scarcity of sarcoid vasculitis in the skin.
Footnotes
All procedures used in this research were approved by the Ethical Committee of all the related institutions.
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Contributor Information
Ko-Ron Chen, Email: chenkr54@yahoo.co.jp.
Keiko Miura, Email: k.miura.pth1@tmd.ac.jp.
Toyoko Inazumi, Email: tinazumi@alles.or.jp.
Yoshio Nakamura, Email: yn1109@z5.keio.jp.
Hideki Nakajima, Email: nakajimahideki2008@gmail.com.
Hayato Takahashi, Email: hayato_takahashi@keio.jp.
Toshiyuki Yamamoto, Email: toyamade@fmu.ac.jp.
REFERENCES
- 1. Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008;31:372–379. [DOI] [PubMed] [Google Scholar]
- 2. Ungprasert P, Ryu JH, Matteson EL. Clinical manifestations, diagnosis, and treatment of sarcoidosis. Mayo Clin Proc Innov Qual Outcomes. 2019;3:358–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2020;41:689–699. [DOI] [PubMed] [Google Scholar]
- 4. Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2007;33:282–286. [DOI] [PubMed] [Google Scholar]
- 5. Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Austral J Dermatol. 2010;51:198–201. [DOI] [PubMed] [Google Scholar]
- 6. Obara K, Maejima H, Mii S, et al. A case of cutaneous sarcoid vasculitis with livedo and review of the literature. Rheumatol Int. 2015;35:195–196. [DOI] [PubMed] [Google Scholar]
- 7. Asahina A, Miura K, Saito I, et al. Cutaneous sarcoidosis with livedoid lesions: evidence of the involvement of propionibacterium acnes. J Dermatol. 2013;40:501–502. [DOI] [PubMed] [Google Scholar]
- 8. Mizuno K, Nguyen CT, Ueda-Hayakawa I, et al. Annular lesions of cutaneous sarcoidosis with granulomatous vasculitis. J Cutan Pathol. 2017;44:494–496. [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto T, Chen KR. Perforating plaque-type pretibial sarcoidosis with granulomatous phlebitis. Am J Dermatopathol. 2020;42:225–226. [DOI] [PubMed] [Google Scholar]
- 10. Yazdani Abyaneh MA, Raghu P, et al. Circumscribed cicatricial alopecia due to localized sarcoidal granulomas and single-organ granulomatous arteritis: a case report and systematic review of sarcoidal vasculitis. J Cutan Pathol. 2015;42:746–756. [DOI] [PubMed] [Google Scholar]
- 11. Endo M, Yamamoto T, Chen KR. Sarcoid vasculitis presenting with erythema nodosum-like lesions. Sarcoidosis Vasc Diffuse Lung Dis. 2021;38:e2021013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aractingi S, Cadranel J, Milleron B, et al. Sarcoidosis associated with leukocytoclastic vasculitis. Dermatology. 1993;187:50–53. [DOI] [PubMed] [Google Scholar]
- 13. Johnston C, Kennedy C. Cutaneous leukocytoclastic vasculitis associated with acute sarcoidosis. Postgrad Med J. 1984;60:549–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Branford WA, Farr PM, Porter DI. Annular vasculitis of the head and neck in a patient with sarcoidosis. Br J Dermatol. 1982;106:713–716. [PubMed] [Google Scholar]
- 15. Miller JA, Johnson M. Mcl: Annular vasculitis in sarcoidosis. Br J Dermatol. 1983;108:123–125. [DOI] [PubMed] [Google Scholar]
- 16. Cecchi R, Giomi A. Annular vasculitis in association with sarcoidosis. J Dermatol. 1999;26:334–336. [DOI] [PubMed] [Google Scholar]
- 17. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 18. Sunderkötter CH, Zelger B, Chen KR, et al. Nomenclature of cutaneous vasculitis: dermatologic addendum to the 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol. 2018;70:171–184. [DOI] [PubMed] [Google Scholar]
- 19. Takemura T, Matsui Y, Oritsu M, et al. Pulmonary vascular involvement in sarcoidosis: granulomatous angiitis and microangiopathy in transbronchial lung biopsies. Virchows Arch A Pathol Anat Histopathol. 1991;418:361–368. [DOI] [PubMed] [Google Scholar]
- 20. Rosen Y, Moon S, Huang CT, et al. Granulomatous pulmonary angiitis in sarcoidosis. Arch Pathol Lab Med. 1977;101:170–174. [PubMed] [Google Scholar]
- 21. Takemura T, Matsui Y, Saiki S, et al. Pulmonary vascular involvement in sarcoidosis: a report of 40 autopsy cases. Human Pathol. 1992;23:1216–1223. [DOI] [PubMed] [Google Scholar]
- 22. Agrawal V, Crisi GM, D’Agati VD, et al. Renal sarcoidosis presenting as acute kidney injury with granulomatous interstitial nephritis and vasculitis. Am J Kidney Dis. 2012;59:303–308. [DOI] [PubMed] [Google Scholar]
- 23. Chen KR, Sakamoto M, Ikemoto K, et al. Granulomatous arteritis in cutaneous lesions of Churg-Strauss syndrome. J Cutan Pathol. 2007;34:330–337. [DOI] [PubMed] [Google Scholar]
- 24. Ishibashi M, Kudo S, Yamamoto K, et al. Churg-Strauss syndrome with coexistence of eosinophilic vasculitis, granulomatous phlebitis and granulomatous dermatitis in bullous pemphigoid-like blisters. J Cutan Pathol. 2011;38:290–294. [DOI] [PubMed] [Google Scholar]
- 25. Carlson JA, Chen KR. Cutaneous vasculitis update: neutrophilic muscular vessel and eosinophilic, granulomatous, and lymphocytic vasculitis syndromes. Am J Dermatopathol. 2007;29:32–43. [DOI] [PubMed] [Google Scholar]
- 26. Gibson LE, el-Azhary RA, Smith TF, et al. The spectrum of cutaneous granulomatous vasculitis: histopathologic report of eight cases with clinical correlation. J Cutan Pathol. 1994;21:437–445. [DOI] [PubMed] [Google Scholar]
- 27. Ishibashi M, Chen KR. A morphological study of evolution of cutaneous polyarteritis nodosa. Am J Dermatopathol. 2008;30:319–326. [DOI] [PubMed] [Google Scholar]
- 28. Morimoto A, Chen KR. Reappraisal of histopathology of cutaneous polyarteritis nodosa. J Cutan Pathol. 2016;43:1131–1138. [DOI] [PubMed] [Google Scholar]