Figure 2.
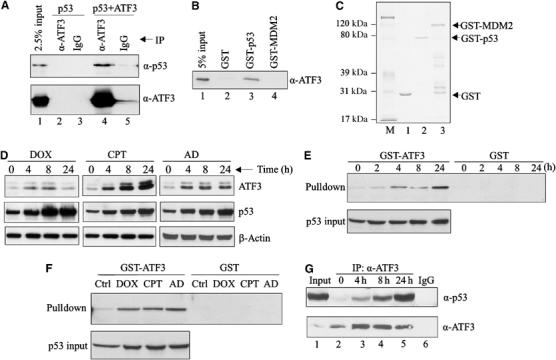
Genotoxic stress increases the direct interaction of the ATF3 and p53 proteins. (A) H1299 cells were transfected with p53 and, where indicated, with pCG/ATF3. Cell lysates (1 mg protein) were incubated with 1 μg of anti-ATF3 antibody or IgG at 4°C overnight, immunocomplexes captured with protein A-agarose and subjected to Western blotting for p53 and ATF3. (B) The indicated GST fusion proteins from bacteria culture were immobilized on glutathione-agarose and incubated with a purified recombinant ATF3 protein 4 h at 4°C. The agarose was extensively washed and eluates subjected to Western blotting for ATF3. (C) The indicated GST fusion proteins were resolved by SDS–PAGE and stained with Coomassie blue. (D) HCT116 cells were treated with 0.4 μg/ml DOX, 2 μM CPT or 5 nM AD as indicated. After the indicated times, cells were lysed and subjected to Western blotting for ATF3 and p53. (E, F) HCT116 cell lysates, adjusted to equal p53 amounts (p53 input), from unstressed and stressed cells (treated as in (D); for (E), the cells were treated with DOX) were incubated with glutathione-agarose beads bound with GST-ATF3 fusion protein or GST protein. After extensive washes, bound material was eluted and immunoblotted for p53. (G) HCT116 cells were treated with 0.4 μg/ml DOX and harvested at the indicated time points. Cell lysates (2 mg protein) were incubated with 0.5 μg ATF3 antibody overnight. Immunoprecipitates were captured and subjected to Western blotting for p53 and ATF3. Lysate from 24-h-treated cells was also immunoprecipitated with 0.5 μg normal IgG as a control (lane 6).
