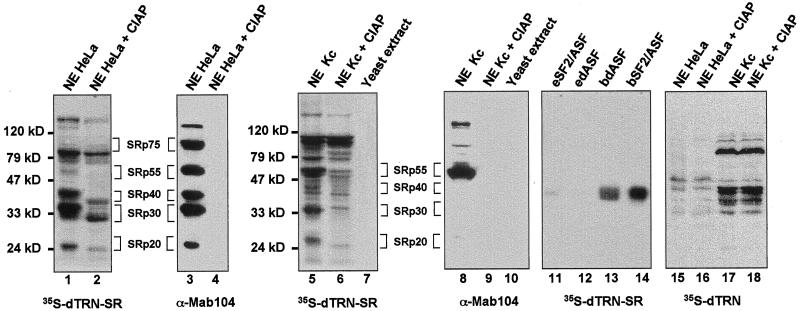
Figure 4.
Far Western analysis showing a physical interaction between dTRN-SR and SR proteins from both HeLa and Drosophila Kc cells. Proteins from HeLa (lanes 1–4, 15, and 16), Drosophila Kc (lanes 5, 6, 8, 9, 17, and 18) nuclear extracts or yeast S. cerevisae total extracts (lanes 7 and 10) either untreated (lanes 1, 3, 5, 7, 8, 10, 15, and 17) or treated (lanes 2, 4, 6, 9, 16, and 18) with calf intestinal alkaline phosphatase (CIAP) as well as purified recombinant dASF and SF2/ASF 100 ng each, expressed either in a baculovirus system (bdASF, lane 13, and bSF2/ASF, lane 14, receptively) or in E. coli (edASF, lane 12, and eSF2/ASF, lane 11, respectively) were separated on a SDS-PAGE, transferred to nitrocellulose, renatured, and probed either with 35-S-labeled dTRN-SR (lanes 1, 2, 5–7, and 11–14) or 35-S-labeled dTRN (lanes 15–18). Proteins (lanes 3, 4, 8, and 9) were subjected to Western blot analysis by using mAb 104. Molecular weight markers are indicated on the left of the Far Western panels, and SR protein species are indicated on the right.