Abstract
Purpose of the Review
Anatomic and reverse endoprosthetic reconstruction are two common surgical options used after tumor resection of the proximal humerus. The purpose of this article is to provide an overview of the functional outcomes and complications of modern anatomic and reverse endoprostheses.
Recent Findings
The anatomic endoprosthesis has traditionally been a successful reconstructive technique as it provided a stable platform upon which the hand and elbow could function. However, the reverse endoprosthesis has gradually replaced the anatomic endoprosthesis given that its semi-constrained design affords greater stability. Patients with reverse endoprostheses have improved motion, patient-reported outcome scores, and revision-free implant survivorship compared to those with anatomic endoprostheses. Shoulder function may be further improved with a reverse allograft prosthetic composite (APC) due to reconstruction of the rotator cuff tendons or by transferring the latissimus dorsi and teres major tendons to recreate the function of the posterosuperior rotator cuff muscles. The short-term functional improvement observed with the use of an allograft reconstruction, however, may diminish with longer follow-up due to delayed graft complications, such as resorption, nonunion, and fracture.
Summary
In most patients undergoing oncologic resection of the proximal humerus, the reverse endoprosthesis or reverse APC is recommended due to improved functional outcomes and reduced postoperative complications compared to other reconstructive techniques.
Keywords: Proximal humeral tumors, Reverse shoulder arthroplasty, Megaprosthesis, Endoprosthesis, Outcomes, Complications
Introduction
The proximal humerus is the third most common site for primary bone sarcomas and metastatic tumors of bone, accounting for 7–15% of all cases [1, 2]. Historically, these tumors were primarily treated with amputation, but there has been an increasing emphasis on limb-sparing resection and shoulder reconstruction [3]. The primary goal of reconstruction is to achieve a stable shoulder to maintain maximum function of the elbow and hand. This can often be challenging due to bone loss and partial resection of the deltoid, rotator cuff muscles, and capsule required to achieve negative margins.
Several reconstructive options are in use today, including arthrodesis, vascularized fibular head autograft, clavicula pro humero, osteoarticular allografts, allograft prosthetic composites (APC), and endoprostheses [4–7]. The evidence supporting one reconstructive option over the other is limited and there is no consensus regarding the optimal reconstructive technique. However, only osteoarticular allografts, APCs, and endoprostheses recreate a glenohumeral articulation that potentially allows for greater shoulder range of motion and function [4]. Osteoarticular allografts have the advantage of replacing bone stock and allows for the direct repair of host rotator cuff tendons to allograft tendon, which may improve shoulder stability and function [8]. Despite these theoretical benefits, it has been shown to be associated with failure rates up to 42% due to graft fracture, resorption, nonunion, and articular deterioration [9]. As such, their use in reconstructing the glenohumeral joint have gradually diminished in favor of endoprostheses and APCs.
Endoprosthetic reconstruction after tumor resection have been utilized since the 1950s [10]. There have been tremendous modifications to their design over the past several decades, particularly with the introduction of the reverse endoprosthesis. In this review, we will highlight the prosthetic reconstruction options available, summarize their clinical outcomes, and provide our preferred treatment option.
Evaluation
The appropriate evaluation of patients with bone or soft tissue sarcomas is predicated on a detailed history and physical exam, imaging, and biopsy. Patients with musculoskeletal malignancy may experience up to a 6-month delay before an accurate diagnosis is made, which may lead to devastating outcomes [11]. They often present with pain, a palpable mass, or pathologic fracture. History should include chronology of symptoms, presence of systemic signs (e.g. weight loss, fevers, fatigue), level of functional independence, personal or family oncologic history, and smoking history. Physical exam should be directed towards evaluating range of motion, deltoid function (axillary nerve), and rotator cuff muscle strength. Focused neurovascular evaluation of the distal arm is critical as the mass may displace the brachial plexus and axillary vessels. A careful examination of the axilla for lymph node enlargement should also be performed.
Further work-up includes orthogonal radiographs of the shoulder and humerus. Location of the lesion and certain radiographic characteristics can help begin to formulate a differential diagnosis. Poor margination, wide zone of transition, periosteal reaction, and cortical disruption are signs of a malignant lesion. In addition, the lesion may have a variety of mineralization patterns that reflect its historical origin (osteoid – cloudlike; chondroid – popcorn, rings or arcs) [12]. A magnetic resonance imaging (MRI) scan with and without gadolinium contrast of the shoulder and entire humerus should also be obtained to evaluate the extent of the lesion, presence of skip metastases, and involvement of the axillary nerve or neurovascular bundle [13]. Lastly, staging studies should include computed tomography (CT) of the chest and positron emission tomography (PET) scan. Any patient over 40 years without a history of cancer who presents with a new lytic bone lesion should be presumed to have metastatic disease until proven otherwise. In addition to a CT chest, a CT abdomen and pelvis should also be obtained to evaluate for the primary lesion [14].
Biopsy in the setting of suspected proximal humeral tumor is a technically challenging procedure that should only be performed at the treating institution. Without the requisite expertise, poorly planned biopsies can compromise the viability of reconstructive procedures and may make amputation necessary to achieve adequate surgical margins [15–17]. Core or open incisional biopsy can be performed to obtain tissue sample, though the diagnostic accuracy of open incisional biopsy is generally higher than that of core biopsy (94% vs. 83%) [17]. Biopsies of the proximal humerus should be performed through the anterior one-third of the deltoid muscle rather than the deltopectoral interval to avoid contamination of other muscles [18]. When the biopsy is collected by open surgery, our preferred technique is to fill the bone with bone wax to limit soft-tissue contamination. The biopsy track should be resected en bloc at the time of reconstruction.
Surgical Planning
Once a tissue diagnosis is confirmed, a multidisciplinary approach that involves radiology, pathology, radiation oncology, medical oncology, and other fields as required, is recommended. If neo-adjuvant chemotherapy is administered, repeat cross-sectional imaging should be performed to determine treatment response and the extent of the tumor. The common belief that neoadjuvant chemotherapy increases the resectability of extremity sarcomas by reducing tumor size, forming a pseudocapsule, and decreasing peritumoral edema has been recently challenged [19]. MRI-planned resections were shown to change meaningfully in inconsistent directions after chemotherapy, suggesting that the planned resection can increase or decrease in size after treatment [19]. We prefer to use T1-weighted sequences to determine the tumor limits, as fluid-sensitive sequences tend to overestimate the tumor limits because of their susceptibility to inflammatory abnormalities [20, 21]. Feathery edema-like pattern seen on T2 imaging has been shown to correspond with tumor-negative areas, whereas bulky or thick edema appearance correlated with the presence of viable tumor cells (Fig. 1) [22]. We generally recommend adding a minimum margin of 20 mm on MRI to allow a safe histological margin.
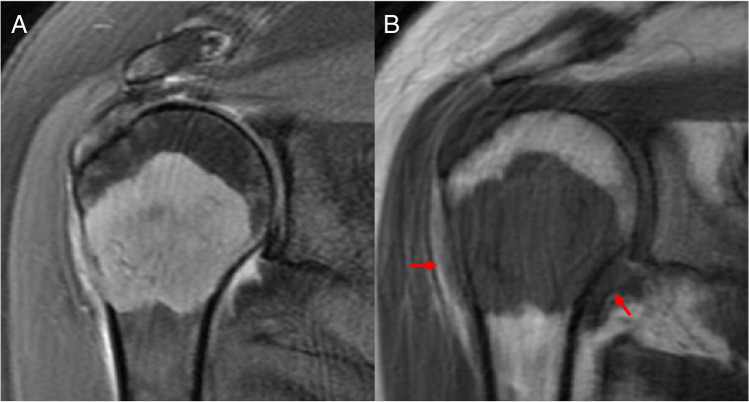
Fig. 1.
Metastatic breast cancer lesion of the proximal humerus (A) T2 fat-saturated imaging demonstrates bulky edema in the joint and along the lateral cortex. B T1-weighted imaging shows likely breach of the lateral cortex. The areas of bulky edema are isointense to the lesion (arrow), which is concerning for viable tumor cells
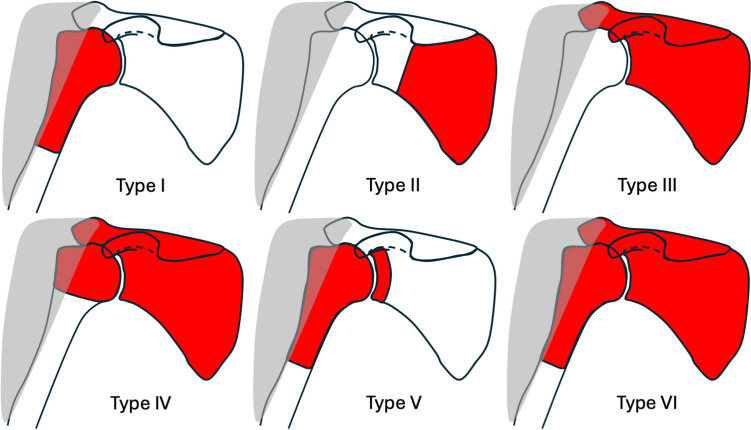
Several classification systems based on the amount of bony resection and status of the deltoid muscle can be used to help guide endoprosthetic reconstruction. The Malawer classification is the most commonly used system and can generally be divided into intra-articular (Type I – III) and extra-articular resections (Type IV – VI) [23]. These resections can be further grouped into A and B depending on the preservation or resection or the deltoid muscle, respectively. Intra-articular spread along the joint capsule and biceps tendon is more common with proximal humeral malignancies than at other sites, which more commonly necessitates an extra-articular resection. Endoprosthetic reconstruction is usually indicated in Malawer Type I and can also be considered in Type V resections if there is sufficient glenoid bone stock (Fig. 2).
Fig. 2.
Malawer classification. The areas highlighted in red are to be resected. Type I and Type V resections are generally amenable to endoprosthetic reconstruction
Endoprosthetic Reconstruction
Anatomic Endoprostheses and APCs
Modular anatomic prostheses were previously the most widely used proximal humerus reconstruction technique due to availability, ease of use, and high implant survival rate [24, 25]. The prosthesis is cemented into the distal humerus and the residual soft tissues are attached to the prosthesis. It can be implanted with or without a functioning abductor mechanism (Malawer Type IA or IB). These prostheses had limited shoulder motion and functioned like a spacer to provide a stable platform for elbow and hand function (Table 1). In an older series of 100 patients, Kumar et al. found that the mean Musculoskeletal Tumor Society (MSTS) score to be 79%, but that shoulder abduction was limited to less than 45° in most patients [26]. Over the past two decades, there has not been significant improvement in range of motion or functional scores despite improvements in implant design [27–29]. Yang et al. found that patients had a mean abduction of 33.5° and MSTS score of 77% [27]. Böhler et al. compared earlier implant designs to newer implant designs and found no significant difference between the mean MSTS score of 73% and 80%, respectively [28]. Not surprisingly, range of motion and functional outcomes were improved with preservation of the deltoid and rotator cuff muscles and axillary nerve.
Table 1.
Studies on anatomic endoprostheses or APCs
| Study | Patients | Mean Follow-up (years) |
ROM (°) | Function | Complications |
|---|---|---|---|---|---|
| Endoprosthesis | |||||
| Cannon 2009 [29] | 83 | 2.5 |
FE: 42 ± 26 Abd: 41 ± 21 |
MSTS: 63% ± 15% |
Instability: 27% Infection: 2% Revision: 2% |
| Raiss 2010 [30] | 39 | 3.2 |
FE: 34 (range, 0–90) Abd: 33 (range, 0–90) ER: 10 (range, 10–50) |
MSTS: 63% (23 − 90%) |
Instability: 10% Infection: 5% Revision: 13% |
| Fujibuchi 2015 [32] | 21 | 1.6 |
Mesh - FE: 65, Abd: 40 No Mesh - FE: 35, Abd: 40 |
NR |
Instability: 0% (mesh) vs. 25% (no mesh) Infection: 5% Revision: 0% |
| Tang 2015 [25] | 29 | 3.8 |
Mesh - FE: 77 ± 26, ER: 73 ± 12 No Mesh - FE: 55 ± 21, ER: 56 ± 9 |
Mesh - MSTS: 79% ± 7%, ASES: 85 ± 11 No Mesh – MSTS: 66% ± 11%, ASES: 72 ± 17 |
Instability: 0% (mesh) vs. 33% (no mesh) Revision: 0% |
| Yang 2021 [27] | 41 | 5 | Abd: 34 (range, 5–71) | MSTS: 77% |
Instability: 5% Aseptic Loosening: 2% Revision: 0% |
| Houdek 2021 [39] | 36 | 7 |
FE: 38 ± 27 ER: 17 ± 14 |
MSTS: 60% ± 13% ASES: 55 ± 16 |
Instability: 37% Infection: 3% Fracture: 6% Revision: 0% |
| APC | |||||
| Abdeen 2009 [34] | 36 | 5 |
FE: 56 (range, 0–150) Abd: 50 (range, 10–140) |
MSTS: 87% (range, 67 − 100%) |
Instability: 17% Delayed Union: 11% Aseptic Loosening: 6% Revision: 8% |
| El Beaino 2019 [37] | 21 | 8 | FE: 92 ± 34 | MSTS: 78% ± 15% |
Instability: 57% Delayed Union: 47% Graft Resorption: 43% Fracture: 5% Revision: 19% |
| Houdek 2021 [39] | 17 | 7 |
FE: 55 ± 31 ER: 21 ± 9 |
MSTS: 69% ± 15% ASES: 60 ± 14 |
Instability: 69% Graft Resorption: 47% Fracture: 18% Revision: 6% |
APC allograft prosthetic composite, ROM range of motion, FE forward elevation, ER external rotation, MSTS Musculoskeletal Tumor Society, ASES American Shoulder Elbow Surgeon, NR none reported
The revision-free implant survivorship of an anatomic proximal humeral endoprosthesis is high, ranging from 70 to 100% at 5-years [1, 5, 26, 30]. The most common complication of the hemiarthroplasty was proximal migration and dislocation, occurring in 26% of patients [24]. This can be attributed to the resection of static and dynamic shoulder stabilizers needed to achieve adequate surgical margins. To minimize the risk of this complication, several authors have described using a synthetic mesh to improve the stability of the prosthesis [25, 31, 32]. In this technique, a polyester or polypropylene mesh is wrapped around the prosthesis and fixed to the remaining glenoid, shoulder girdle, or rotator cuff tendons with nonabsorbable sutures [25, 32]. In a comparative study between modular anatomic prostheses with and without the use of synthetic mesh, the addition of a synthetic mesh was found to significantly improve mean shoulder abduction (77° vs. 55°) and MSTS score (79% vs. 66%) [25]. Furthermore, none of the patients with synthetic mesh reconstruction had proximal migration or prosthetic dislocation [25]. It should be noted that patients with endoprosthesis subluxation were historically not revised due to concern for repeat subluxation events secondary to lack of soft-tissue attachments [24]. Recent studies, however, have shown that revision with a reverse APC has been shown to effectively restore shoulder stability, range of motion, and function in these patients [33].
An anatomic APC is one alternative to an anatomic endoprosthesis for proximal humeral reconstruction. It usually involves cementing a long-stem hemiarthroplasty into a proximal humeral allograft, which is subsequently fixed to host bone using compression plating. This technique combines the potential advantages of an allograft soft-tissue tendinous and capsular attachments with the benefits of a humeral prosthesis. Abdeen et al. reported on a series of 36 patients who underwent anatomic APC and found a mean abduction of 50° and MSTS score of 87% with implant survivorship of 88% at 5-years [34]. Some studies have demonstrated similar functional results and survivorship, whereas others have not [35–37]. El Beaino et al. found that the MSTS scores significantly deteriorated from 86% at 1-year follow-up to 78% at 5-year follow-up [37]. The authors surmised that a variety of delayed complications, such as resorption of the greater tuberosity and subsequent superior migration due to rotator cuff insufficiency [37]. Complications associated with this reconstructive technique were common and included instability (34%), allograft resorption (19%), nonunion (6%), and graft fracture/hardware failure (10%) [24].
Reverse Endoprostheses and APCs
Wide resection of the proximal humerus often necessitates removal of a portion of the rotator cuff tendons. This makes the reverse endoprosthesis an ideal option for oncologic reconstruction because its semi-constrained design is inherently stable and resists proximal humeral migration. Furthermore, the reverse prosthesis increases the moment arm of the deltoid, allowing adequate power for elevation in the absence of a functional rotator cuff. Several comparative studies have demonstrated significantly better motion and functional outcome scores among patients who underwent a reverse endoprosthesis than patients who underwent an anatomic endoprosthesis (Table 2) [38–41]. In a retrospective series, Houdek et al. found that patients with reverse endoprosthesis had significantly better active forward elevation (76° vs. 38°), external rotation (27° vs. 17°), and MSTS scores (67% vs. 60%) than that of anatomic endoprostheses, but there was no difference in ASES scores or satisfaction rates [39]. Moreover, no patient with a reverse endoprosthesis had instability compared to 37% of patients with an anatomic endoprosthesis. Among patients with nonprimary malignancies of the proximal humerus, Sullivan et al. also showed that reverse endoprostheses had superior forward elevation (74° vs. 32°), ASES (63 vs. 57), and MSTS (67% vs. 60%) scores [38]. Trikoupis et al. found similar results, demonstrating a significantly higher 5-year implant survivorship (87% vs. 68%) and improved range of motion and patient-reported outcomes in patients with reverse endoprostheses [41]. Systematic reviews and meta-analyses have also shown superior functional outcomes with a reduction in complications when a reverse endoprosthesis is used compared to an anatomic endoprosthesis [24, 42, 43]. As such, reconstruction using a reverse prosthesis has become our preferred technique following oncologic resection of the proximal humerus (Fig. 2).
Table 2.
Studies on reverse endoprostheses or APCs
| Study | Patients | Mean Follow-up (years) | ROM (°) | Function | Complications |
|---|---|---|---|---|---|
| Endoprosthesis | |||||
| Streitbuerger 2015 [44] | 18 | 2.8 |
FE: 84 (range, 30–160) ER: 35 (range, 15–50) |
MSTS: 82% ± 11% |
Instability: 22% Infection: 6% Revision: 6% |
| Trovarelli 2019 [45] | 22 | 3 |
FE: 117 (range, 40–180) Abd: 103 (range, 40–180) ER: 58 (range, 45–75) |
MSTS: 97% (87% − 100%) ASES: 81 (range, 62–92) Constant: 61 (range, 42–89) |
Instability: 18% Aseptic Loosening: 5% Revision: 23% |
| Houdek 2021 [39] | 20 | 7.4 |
FE: 76 ± 38 ER: 27 ± 15 |
MSTS: 67% ± 10% ASES: 61 ± 13 |
Instability: 0% Infection: 5% Revision: 0% |
| Trikoupis 2022 [41] | 19 | 5.2 |
FE: 90 ± 15 Abd: 80 ± 11 |
MSTS: 76% ± 8% |
Instability: 5% Infection: 5% Aseptic Loosening: 5% Revision: 10% |
| APC | |||||
| Houdek 2021 [39] | 10 | 7.4 |
FE: 100 ± 39 ER: 34 ± 11 |
MSTS: 80% ± 9% ASES: 72 ± 10 |
Instability: 0% Graft Resorption: 60% Graft Fracture: 20% Revision: 0% |
| Callamand 2022 [49] | 11 | 2.5 |
FE: 105 (range, 30–160) ER: 23 (range, −20–80) |
Constant: 49 (range, 24–75) SSV: 52% (range, 30%–75%) |
Instability: 9% Graft Resorption: 64% Revision: 9% |
APC allograft prosthetic composite, ROM range of motion, FE forward elevation, ER external rotation, MSTS Musculoskeletal Tumor Society, ASES American Shoulder Elbow Surgeon, SSV Subjective Shoulder Value
Although the reverse semi-constrained design affords greater stability, the most common complication continues to be instability [24, 43]. In a case series of 18 patients who underwent a reverse endoprosthesis, Streitbuerger et al. reported that 4 (22%) patients had shoulder luxation, of which two resolved with intensive physiotherapy focused on strengthening the remaining shoulder musculature [44]. Similarly, in a study of patients with preserved deltoid function, Trovarelli et al. had 4 (18%) patients with dislocations at a mean time of 1 month that all required revision arthroplasty [45]. The main reason for dislocation was attributed to inadequate deltoid tension especially in longer resections that require detachment and reattachment of the deltoid [45]. Several systematic reviews have estimated the instability rate to be approximately 17–18% [24, 43]. Multiple surgical strategies can be employed to improve the stability of the prosthesis in the setting of inadequate soft tissue tensioning. Increased tension and deltoid wrapping can be achieved by using an implant with a larger proximal body that replicates the greater tuberosity or an APC [46]. Glenohumeral or acromiohumeral cerclage can also be utilized for recalcitrant instability by using multiple high-strength sutures to stabilize the humerus to either the glenoid or acromion, respectively [47, 48].
Another reconstructive option for proximal humeral resections is the reverse APC. This reconstruction provides inherent stability from the reverse implants while also allowing for bone stock restoration and soft tissue reconstruction. The cadaveric rotator cuff tendons can be directly repaired to the host rotator cuff, potentially improving shoulder range of motion [39]. Moreover, active forward elevation and external rotation may also be further improved by transferring the latissimus dorsi and teres major tendons to the posterolateral aspect of the humeral allograft (L’Episcopo procedure) [46]. At short-term follow-up, Houdek et al. found that patients with reverse APC had significantly better active forward elevation (100° vs. 76°), ASES (72 vs. 61), and MSTS (80% vs. 67%) scores than patients with a reverse endoprosthesis [39]. Similarly, Callamand et al. reported a mean active forward elevation of 105° and external rotation of 23° in 11 patients with a reverse APC at 2.5 year follow-up [49]. The L’Episcopo transfer was also shown to further increase active forward elevation (140° vs. 75°) and external rotation (38° vs. 7°) [49]. Despite the promising initial results with reverse APCs, larger and long term follow-up studies are needed to determine if reverse APCs are superior to reverse endoprostheses. Complications associated with the allograft, such as resorption of the tuberosities and tendon attachments, may lead to a deterioration of functional results and instability as seen with anatomic APCs [37].
Traditionally, a nonfunctional deltoid due to axillary nerve or massive deltoid muscle resection was a contraindication to a reverse endoprosthesis. The Bayley-Walker prosthesis is a highly constrained fixed-fulcrum reverse shoulder replacement that was designed to resist instability due to loss of stabilizing structures and has been in use since 1994 [50]. The prosthesis features a highly conforming humeral component that almost circumferentially envelops the glenosphere. Early small case series with demonstrated good results with 71° of forward elevation, no dislocations, and 100% revision-free survivorship at mean follow-up of 4 years [51]. However, more recent studies showed high rates of constrained mechanism failure (26%) or aseptic loosening (18%) without any functional benefit [50, 52].
Senior Authors’ Preferred Technique
At our institution, a multidisciplinary approach is utilized where the orthopaedic oncology surgeon resects the tumor and the shoulder-trained surgeon performs the reconstruction. We prefer to utilize reverse endoprosthesis as it provides superior functional outcomes when compared to an anatomic endoprosthesis [42, 43]. Furthermore, it is likely associated with longer implant survivorship compared to reverse APC due to avoidance of graft-related complications [37, 53].
Approach and Tumor Resection
The patient is positioned in the beach-chair with the entire arm prepared. The skin incision incorporates and removes the prior biopsy site. The approach is carried through the deltopectoral interval, excising a portion of the anterior deltoid that was previously used for the biopsy tract. The extent of the resection is dictated by the pre-operative imaging and the extent of the tumor. The long head of biceps tendon is cut distally so that it is removed with the proximal humerus. The pectoralis major tendon insertion is then released, leaving a small cuff on the bone for margins. The latissimus dorsi and teres major are similarly divided. The tendons are tagged with nonabsorbable suture for later reattachment to the prosthesis at the end of the procedure. The anterior circumflex vessels are suture ligated. The subscapularis and supraspinatus tendons are divided at the musculotendinous junction, ensuring an extra-capsular approach to the joint. The shoulder is then gradually dislocated by releasing the inferior and posterior capsule and soft tissues. The level of the planned humeral osteotomy is then carefully measured from the greater tuberosity or pectoralis major insertion if the tuberosity is affected based on preoperative imaging. If the planned resection is below the level of the deltoid insertion, the deltoid is detached from the humerus, preserving its aponeurosis and fascia so that it can be reattached after reconstruction. A transverse humeral osteotomy is then made. The proximal humeral tumor specimen is then released from any remaining posterior soft tissues. It is oriented, marked, and sent to pathology for fresh frozen section. The marrow of the distal cut is also sent for fresh frozen section to ensure negative margins. Any further areas that appear suspicious of possible malignancy are also sent for laboratory examination. Once the pathologist confirms that the margins are negative, all gowns and gloves are changed to minimize the risk of tumor spread. A new back table containing all reconstruction instruments is used.
Glenoid Reconstruction
The glenoid is carefully exposed as the bone quality may be poor due to inactivity or chemotherapy. In Malawer Type I resections (intra-articular), the glenoid is reamed to achieve neutral version and tilt. The baseplate is placed flush with the inferior glenoid rim to maximize impingement-free range of motion. A large diameter glenosphere is used to decrease the risk of dislocation. We generally aim to place the center of rotation at the patient’s native joint line. In Malawer Type 5 resections (extra-articular), the glenoid needs to be reconstructed to restore the center of rotation to the native joint line. At least 10–15 mm of the glenoid vault is recommended to allow for sufficient fixation of the glenoid component [54]. Our preferred option to achieve the lateralization is through the metallic baseplate and glenosphere. In situations where a significant amount of the glenoid vault is planned to be resected, we elect to use a custom baseplate. Other previously described techniques include bony-increased offset reverse shoulder arthroplasty with use of iliac crest autograft or allograft [55].
Humeral Reconstruction
Several important parameters, including humeral length, retroversion, and fixation, require special consideration in reverse endoprosthetic reconstruction. Humeral length should be restored to the length measured before the resection to allow sufficient tensioning of the soft tissues. A modular endoprosthesis is used as it provides the surgeon more flexibility in altering length by modifying the intercalary segment, proximal body, or tray. Monobloc humeral stems are technically more challenging to implant, but avoid the risk of intra-component disassembly. The humeral trial is placed in 20° of retroversion as this has been previously described to have the greatest impingement-free external rotation with the shoulder adducted [56, 57]. The trial is then reduced with an appropriately sized polyethylene liner, ensuring excellent tensioning of the deltoid. For resections that continue to have a supportive diaphyseal isthmus after reaming, components are press fit (Fig. 3). However, for older patients with capacious canals and thin cortices, components are cemented (Fig. 4). In select cases where the resection leaves a non-supportive isthmus, compressive osseointegration is used (Fig. 5). This technology provides secure, long-term anchorage by using a spring-loaded device to achieve compliant prestress fixation. Results in lower extremity endoprosthesis have demonstrated progressive bone hypertrophy at the prosthetic interface and excellent survivorship [58]. A polyethylene terephthalate mesh is secured around the humeral implant with nonabsorbable sutures to facilitate soft-tissue healing around the prosthesis. The pectoralis major and deltoid, if detached due to a long resection, are sutured to their anatomic insertions on the mesh and implant. The latissimus dorsi and teres major tendons are similarly sutured to the posterolateral aspect of the implant to potentially increase forward elevation and external rotation.
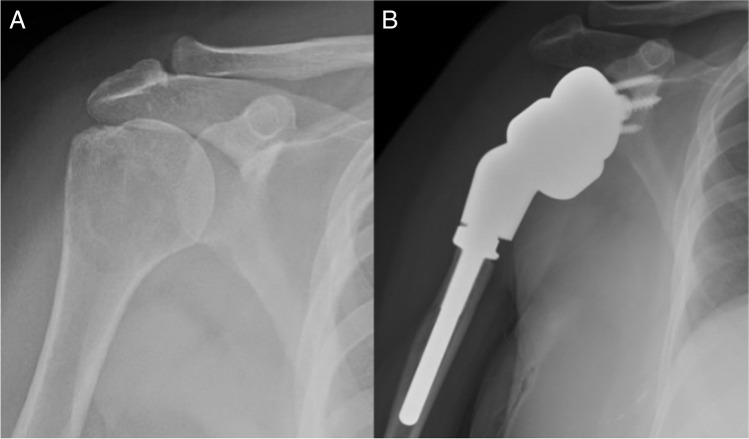
Fig. 3.
48 year-old female with (A) metastatic breast cancer lesion of the proximal humerus. B Cementless reverse endoprosthesis at 1-year follow-up
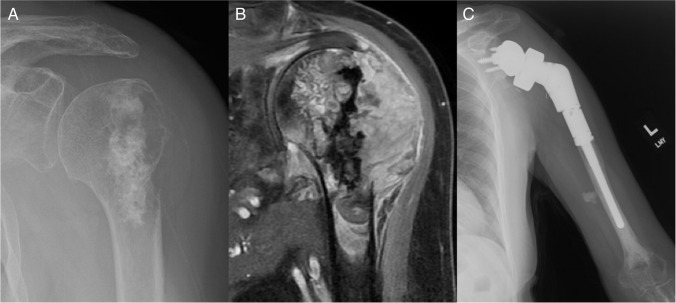
Fig. 4.
69 year-old female with (A) dedifferentiated chondrosarcoma of the proximal humerus with extension into the subdeltoid space. B T1-weighted image with contrast shows a large expansile lesion without extension into the glenoid. C Cemented reverse endoprosthesis at 1-year follow-up
Fig. 5.
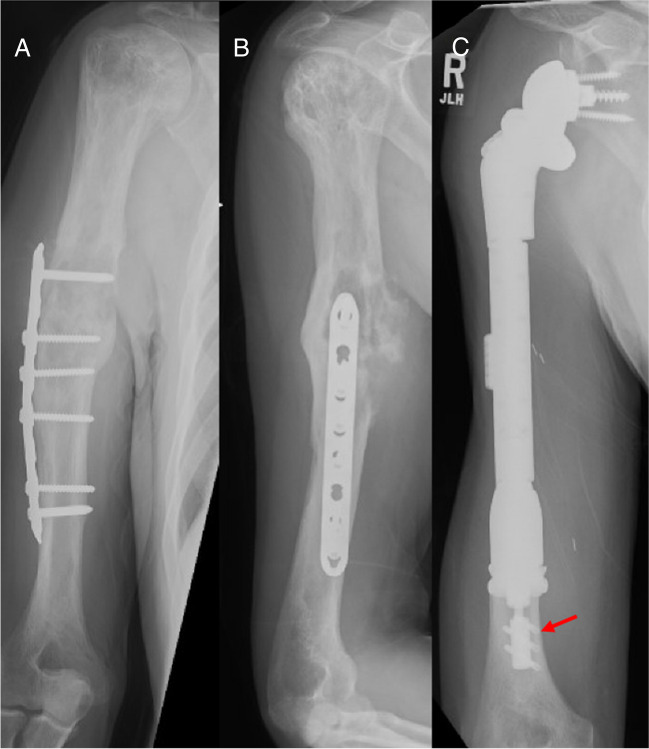
63 year-old male with (A and B) undifferentiated pleomorphic sarcoma extending from the humeral shaft to the proximal humerus. The patient sustained a prior humeral shaft fracture that was treated with plate fixation. C Reverse endoprosthesis with compressive osseointegration technique (arrow) used to achieve fixation in the distal humeral shaft
Postoperative Care
A 1-day regimen of postoperative intravenous cephalosporin is used for surgical prophylaxis [59]. Patients are placed in a shoulder immobilizer for 6 weeks to protect the soft tissue repairs. After 6 weeks, patients are gradually transitioned from passive to active range of motion. Strengthening is initiated 3 months after surgery. From an oncologic perspective, patients with bone sarcomas are followed with radiographs, metal-suppressed MRI with and without contrast, and CT chest to evaluate for disease recurrence [60]. This is performed every 3 months for the first two years, every 6 months until 5-years, and yearly until 10-years.
Conclusion
The anatomic endoprosthesis has traditionally been a successful reconstructive option after tumor resection of the proximal humerus as it provided a stable platform upon which the hand and elbow could function. However, the reverse endoprosthesis has gradually replaced the anatomic endoprosthesis given that its semi-constrained design affords greater stability. Moreover, there is improved motion, patient-reported outcome scores, and revision-free implant survivorship in patients with a reverse endoprosthesis compared to those with an anatomic endoprosthesis. Shoulder function may be further improved with a reverse APC due to reconstruction of the rotator cuff tendons or by performing a tendon transfer to recreate the function of the posterosuperior rotator cuff muscles. Despite promising results, neither the reverse endoprosthesis nor the reverse APC has long-term or large data and continued surveillance of their performance is necessary.
Key References
- Tang X, Guo W, Yang R, Tang S, Ji T. Synthetic mesh improves shoulder function after intraarticular resection and prosthetic replacement of proximal humerus. Clin Orthop Relat Res. 2015;473(4):1464-71. https://doi.org/10.1007/s11999-015–4139−7 .
- Retrospective study that showed patients with anatomic endoprosthesis with mesh had improved motion and function and reduced rates of instability compared to those without mesh.
- Abdeen A, Hoang BH, Athanasian EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91(10):2406-15. https://doi.org/10.2106/JBJS.H.00815.
- This early study of anatomic APCs showed that this reconstruction technique is at least as good as osteoarticular allografts and endoprostheses. Motion was directly correlated with the amount of deltoid muscle preservation. Implant survivorship at 10-years was 88%.
- El Beaino M, Liu J, Lewis VO, Lin PP. Do Early Results of Proximal Humeral Allograft-Prosthetic Composite Reconstructions Persist at 5-year Followup? Clin Orthop Relat Res. 2019;477(4):758-65. https://doi.org/10.1097/CORR.0000000000000354 .
- This retrospective study showed that the functional outcomes of anatomic APCs began to deteriorate from 1- to 5-year follow-up. Delayed graft complications, including graft resorption and superior migration and nonunion, occurred frequently and may contribute to the diminishing scores.
- Houdek MT, Bukowski BR, Athey AG, Elhassan BT, Barlow JD, Morrey ME et al. Comparison of reconstructive techniques following oncologic intraarticular resection of proximal humerus. J Surg Oncol. 2021;123(1):133-40. https://doi.org/10.1002/jso.26271.
- Comparative study highlighting the improved functional outcome in patients undergoing reconstruction with a reverse arthroplasty compared to traditional anatomic prosthesis. Specifically, reverse APCs had the greatest functional improvement, outperforming even reverse endoprostheses.
- Trovarelli G, Cappellari A, Angelini A, Pala E, Ruggieri P. What Is the Survival and Function of Modular Reverse Total Shoulder Prostheses in Patients Undergoing Tumor Resections in Whom an Innervated Deltoid Muscle Can Be Preserved? Clin Orthop Relat Res. 2019;477(11):2495-507. https://doi.org/10.1097/CORR.0000000000000899.
- Among patients with preserved deltoid function, instability after reverse endoprosthesis can still be as high as 18%.
- Callamand G, Barret H, Saint-Genez F, Bonnevialle P, Mansat P, Bonnevialle N. Reconstruction by allograft-prosthetic composite reverse shoulder arthroplasty after proximal humerus tumor resection: Clinical and radiographic assessment at a minimum 2years’ follow-up. Orthop Traumatol Surg Res. 2022;108(4):102957. https://doi.org/10.1016/j.otsr.2021.102957.
- Retrospective case series of 11 patients with reverse APCs with and without L’Episcopo tendon transfer. The tendon transfer sign.
Author Contributions
Favian Su – Literature search, data collection, writing – original draft preparation; Edgar Garcia-Lopez – Literature search, data collection, writing – original draft preparation; Rosanna Wustrack – Conceptualization, writing – reviewing and editing; Drew A. Lansdown – Conceptualization, writing – reviewing and editing.
Funding
No funds, grants, or other support was received.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Potter BK, Adams SC, Pitcher JD Jr., Malinin TI, Temple HT. Proximal humerus reconstructions for tumors. Clin Orthop Relat Res. 2009;467(4):1035–41. 10.1007/s11999-008-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DH, Hills JM, Jordanov MI, Jaffe KA. Common tumors and tumor-like lesions of the shoulder. J Am Acad Orthop Surg. 2019;27(7):236–45. 10.5435/JAAOS-D-17-00449. [DOI] [PubMed] [Google Scholar]
- 3.Malawer M, Sugarbaker PH. Musculoskeletal Cancer surgery. Springer, Dordrecht; 2001. [Google Scholar]
- 4.Teunis T, Nota SP, Hornicek FJ, Schwab JH, Lozano-Calderon SA. Outcome after reconstruction of the proximal humerus for tumor resection: a systematic review. Clin Orthop Relat Res. 2014;472(7):2245–53. 10.1007/s11999-014-3474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodl RW, Gosheger G, Gebert C, Lindner N, Ozaki T, Winkelmann W. Reconstruction of the proximal humerus after wide resection of tumours. J Bone Joint Surg Br. 2002;84(7):1004–8. 10.1302/0301-620x.84b7.12989. [DOI] [PubMed] [Google Scholar]
- 6.Barbier D, De Billy B, Gicquel P, Bourelle S, Journeau P. Is the Clavicula Pro Humero technique of Value for Reconstruction after Resection of the proximal humerus in children? Clin Orthop Relat Res. 2017;475(10):2550–61. 10.1007/s11999-017-5438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada T, Usui M, Isu K, Yamawakii S, Ishii S. Reconstruction and limb salvage after resection for malignant bone tumour of the proximal humerus. A sling procedure using a free vascularised fibular graft. J Bone Joint Surg Br. 1999;81(5):808–13. 10.1302/0301-620x.81b5.9430. [DOI] [PubMed] [Google Scholar]
- 8.Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81(8):1138–46. 10.2106/00004623-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Bus MP, van de Sande MA, Taminiau AH, Dijkstra PD. Is there still a role for osteoarticular allograft reconstruction in musculoskeletal tumour surgery? A long-term follow-up study of 38 patients and systematic review of the literature. Bone Joint J. 2017;99–B(4):522–30. 10.1302/0301-620X.99B4.BJJ-2016-0443.R2. [DOI] [PubMed] [Google Scholar]
- 10.Ross AC, Wilson JN, Scales JT. Endoprosthetic replacement of the proximal humerus. J Bone Joint Surg Br. 1987;69(4):656–61. 10.1302/0301-620X.69B4.3611177. [DOI] [PubMed] [Google Scholar]
- 11.Brouns F, Stas M, De Wever I. Delay in diagnosis of soft tissue sarcomas. Eur J Surg Oncol. 2003;29(5):440–5. 10.1016/s0748-7983(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 12.Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology. 2008;246(3):662–74. 10.1148/radiol.2463061038. [DOI] [PubMed] [Google Scholar]
- 13.Saifuddin A, Sharif B, Oliveira I, Kalus S, Barnett J, Pressney I. The incidence of skip metastases on whole bone MRI in high-grade bone sarcomas. Skeletal Radiol. 2020;49(6):945–54. 10.1007/s00256-019-03369-9. [DOI] [PubMed] [Google Scholar]
- 14.Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. A prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75(9):1276–81. 10.2106/00004623-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Trieu J, Sinnathamby M, Di Bella C, Pianta M, Perera W, Slavin JL, et al. Biopsy and the diagnostic evaluation of musculoskeletal tumours: critical but often missed in the 21st century. ANZ J Surg. 2016;86(3):133–8. 10.1111/ans.13251. [DOI] [PubMed] [Google Scholar]
- 16.Mankin HJ, Lange TA, Spanier SS. The Classic : The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. The Journal of Bone and Joint Surgery, 1982;64:1121–1127. Clin Orthop Relat Res. 2006;450:4–10. 10.1097/01.blo.0000229299.36969.b5. [DOI] [PubMed]
- 17.Rougraff BT, Aboulafia A, Biermann JS, Healey J. Biopsy of soft tissue masses: evidence-based medicine for the musculoskeletal tumor society. Clin Orthop Relat Res. 2009;467(11):2783–91. 10.1007/s11999-009-0965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meek RD, Mills MK, Hanrahan CJ, Beckett BR, Leake RL, Allen H, et al. Pearls and pitfalls for soft-tissue and bone biopsies: a cross-institutional review. Radiographics. 2020;40(1):266–90. 10.1148/rg.2020190089. [DOI] [PubMed] [Google Scholar]
- 19.Jones KB, Ferguson PC, Lam B, Biau DJ, Hopyan S, Deheshi B, et al. Effects of neoadjuvant chemotherapy on image-directed planning of surgical resection for distal femoral osteosarcoma. J Bone Joint Surg Am. 2012;94(15):1399–405. 10.2106/JBJS.K.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thevenin-Lemoine C, Destombes L, Vial J, Wargny M, Bonnevialle P, Lefevre Y, et al. Planning for bone excision in Ewing Sarcoma: Post-chemotherapy MRI more Accurate Than Pre-chemotherapy MRI Assessment. J Bone Joint Surg Am. 2018;100(1):13–20. 10.2106/JBJS.16.01461. [DOI] [PubMed] [Google Scholar]
- 21.Panuel M, Gentet JC, Scheiner C, Jouve JL, Bollini G, Petit P, et al. Physeal and epiphyseal extent of primary malignant bone tumors in childhood. Correlation of preoperative MRI and the pathologic examination. Pediatr Radiol. 1993;23(6):421–4. 10.1007/BF02012438. [DOI] [PubMed] [Google Scholar]
- 22.Masrouha KZ, Musallam KM, Samra AB, Tawil A, Haidar R, Chakhachiro Z, et al. Correlation of non-mass-like abnormal MR signal intensity with pathological findings surrounding pediatric osteosarcoma and Ewing’s sarcoma. Skeletal Radiol. 2012;41(11):1453–61. 10.1007/s00256-012-1383-8. [DOI] [PubMed] [Google Scholar]
- 23.Malawer MM. Tumors of the shoulder girdle. Technique of resection and description of a surgical classification. Orthop Clin North Am. 1991;22(1):7–35. [PubMed] [Google Scholar]
- 24.Aiba H, Atherley O’Meally A, Aso A, Tsukamoto S, Kimura H, Murakami H, et al. Malawer type I/V proximal humerus reconstruction after tumor resection: a systematic review. J Shoulder Elb Surg. 2024;33(9):2096–108. 10.1016/j.jse.2024.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Tang X, Guo W, Yang R, Tang S, Ji T. Synthetic mesh improves shoulder function after intraarticular resection and prosthetic replacement of proximal humerus. Clin Orthop Relat Res. 2015;473(4):1464–71. 10.1007/s11999-015-4139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar D, Grimer RJ, Abudu A, Carter SR, Tillman RM. Endoprosthetic replacement of the proximal humerus. Long-term results. J Bone Joint Surg Br. 2003;85(5):717–22. [PubMed] [Google Scholar]
- 27.Yang Y, Li Y, Liu W, Niu X. Mesh patch and anchors can improve clinical results of prosthetic replacement after resection of primary proximal humerus malignant tumor. Sci Rep. 2021;11(1):734. 10.1038/s41598-020-78959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohler C, Bronimann S, Kaider A, Puchner SE, Sigmund IK, Windhager R, et al. Surgical and Functional Outcome after Endoprosthetic Reconstruction in patients with Osteosarcoma of the Humerus. Sci Rep. 2018;8(1):16148. 10.1038/s41598-018-34397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannon CP, Paraliticci GU, Lin PP, Lewis VO, Yasko AW. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elb Surg. 2009;18(5):705–10. 10.1016/j.jse.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Raiss P, Kinkel S, Sauter U, Bruckner T, Lehner B. Replacement of the proximal humerus with MUTARS tumor endoprostheses. Eur J Surg Oncol. 2010;36(4):371–7. 10.1016/j.ejso.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Ji T, Tang X, Guo W. Enhancing soft-tissue reattachment in proximal humeral endoprosthetic reconstruction. J Orthop Surg (Hong Kong). 2014;22(1):100–3. 10.1177/230949901402200125. [DOI] [PubMed] [Google Scholar]
- 32.Fujibuchi T, Matsumoto S, Shimoji T, Ae K, Tanizawa T, Gokita T, et al. New endoprosthesis suspension method with polypropylene monofilament knitted mesh after resection of bone tumors in proximal humerus. J Shoulder Elb Surg. 2015;24(6):882–8. 10.1016/j.jse.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Houdek MT, Wagner ER, Rose PS, Barlow JD, Elhassan BT, Sanchez-Sotelo J. Allograft prosthetic composite reconstruction using a reverse total shoulder arthroplasty for failed oncologic proximal humerus reconstruction. J Surg Oncol. 2022;125(4):775–81. 10.1002/jso.26772. [DOI] [PubMed] [Google Scholar]
- 34.Abdeen A, Hoang BH, Athanasian EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91(10):2406–15. 10.2106/JBJS.H.00815. [DOI] [PubMed] [Google Scholar]
- 35.Nota S, Teunis T, Kortlever J, Ferrone M, Ready J, Gebhardt M, et al. Functional outcomes and complications after Oncologic Reconstruction of the proximal Humerus. J Am Acad Orthop Surg. 2018;26(11):403–9. 10.5435/JAAOS-D-16-00551. [DOI] [PubMed] [Google Scholar]
- 36.Ogink PT, Teunissen FR, Massier JR, Raskin KA, Schwab JH, Lozano-Calderon SA. Allograft reconstruction of the humerus: complications and revision surgery. J Surg Oncol. 2019;119(3):329–35. 10.1002/jso.25309. [DOI] [PubMed] [Google Scholar]
- 37.El Beaino M, Liu J, Lewis VO, Lin PP. Do early results of Proximal Humeral Allograft-Prosthetic Composite reconstructions Persist at 5-year Followup? Clin Orthop Relat Res. 2019;477(4):758–65. 10.1097/CORR.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan MH, Arguello AM, Barlow JD, Morrey ME, Rose PS, Sanchez-Sotelo J, et al. Comparison of reconstructive techniques for nonprimary malignancies in the proximal humerus. J Surg Oncol. 2024;130(1):64–71. 10.1002/jso.27693. [DOI] [PubMed] [Google Scholar]
- 39.Houdek MT, Bukowski BR, Athey AG, Elhassan BT, Barlow JD, Morrey ME, et al. Comparison of reconstructive techniques following oncologic intraarticular resection of proximal humerus. J Surg Oncol. 2021;123(1):133–40. 10.1002/jso.26271. [DOI] [PubMed] [Google Scholar]
- 40.Grosel TW, Plummer DR, Everhart JS, Kirven JC, Ziegler CL, Mayerson JL, et al. Reverse total shoulder arthroplasty provides stability and better function than hemiarthroplasty following resection of proximal humerus tumors. J Shoulder Elb Surg. 2019;28(11):2147–52. 10.1016/j.jse.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 41.Trikoupis IG, Savvidou OD, Tsantes AG, Papadopoulos DV, Goumenos SD, Vottis C, et al. Prosthetic Reconstruction of the Shoulder after Resection of Proximal Humerus Bone Tumor. Orthopedics. 2022;45(6):e335–41. 10.3928/01477447-20220907-03. [DOI] [PubMed] [Google Scholar]
- 42.Fiore M, Sambri A, Giannini C, Zucchini R, De Cristofaro R, De Paolis M. Anatomical and reverse megaprosthesis in proximal humerus reconstructions after oncologic resections: a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2022;142(10):2459–69. 10.1007/s00402-021-03857-5. [DOI] [PubMed] [Google Scholar]
- 43.Ferlauto HR, Wickman JR, Lazarides AL, Hendren S, Visgauss JD, Brigman BE, et al. Reverse total shoulder arthroplasty for oncologic reconstruction of the proximal humerus: a systematic review. J Shoulder Elb Surg. 2021;30(11):647–58. 10.1016/j.jse.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Streitbuerger A, Henrichs M, Gosheger G, Ahrens H, Nottrott M, Guder W, et al. Improvement of the shoulder function after large segment resection of the proximal humerus with the use of an inverse tumour prosthesis. Int Orthop. 2015;39(2):355–61. 10.1007/s00264-014-2560-2. [DOI] [PubMed] [Google Scholar]
- 45.Trovarelli G, Cappellari A, Angelini A, Pala E, Ruggieri P. What is the survival and function of Modular Reverse Total Shoulder prostheses in patients undergoing Tumor resections in whom an innervated deltoid muscle can be preserved? Clin Orthop Relat Res. 2019;477(11):2495–507. 10.1097/CORR.0000000000000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boileau P, Raynier JL, Chelli M, Gonzalez JF, Galvin JW. Reverse shoulder-allograft prosthesis composite, with or without tendon transfer, for the treatment of severe proximal humeral bone loss. J Shoulder Elb Surg. 2020;29(11):e401–15. 10.1016/j.jse.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 47.Tashjian RZ, Broschinsky K, Chalmers PN. Glenohumeral cerclage for salvage of recalcitrant instability after reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2018;27(8):e259–63. 10.1016/j.jse.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Salazar DH, Bialek SE, Garbis NG. Acromiohumeral cerclage in reverse total shoulder arthroplasty for recurrent instability. J Shoulder Elb Surg. 2022;31(8):e376–85. 10.1016/j.jse.2022.01.136. [DOI] [PubMed] [Google Scholar]
- 49.Callamand G, Barret H, Saint-Genez F, Bonnevialle P, Mansat P, Bonnevialle N. Reconstruction by allograft-prosthetic composite reverse shoulder arthroplasty after proximal humerus tumor resection: clinical and radiographic assessment at a minimum 2years’ follow-up. Orthop Traumatol Surg Res. 2022;108(4):102957. 10.1016/j.otsr.2021.102957. [DOI] [PubMed] [Google Scholar]
- 50.Cundy WJ, McArthur MS, Dickinson IC, Rowell PD, Sommerville SMM. Constrained or unconstrained shoulder replacement for musculoskeletal tumor resections? J Shoulder Elb Surg. 2020;29(10):2104–10. 10.1016/j.jse.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Maclean S, Malik SS, Evans S, Gregory J, Jeys L. Reverse shoulder endoprosthesis for pathologic lesions of the proximal humerus: a minimum 3-year follow-up. J Shoulder Elbow Surg. 2017;26(11):1990–4. 10.1016/j.jse.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Ayvaz M, Cetik RM, Bakircioglu S, Tokgozoglu AM. Proximal Humerus tumors: higher-than-expected risk of Revision with constrained reverse shoulder arthroplasty. Clin Orthop Relat Res. 2020;478(11):2585–95. 10.1097/CORR.0000000000001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Houdek MT, Sullivan MH, Broida SE, Barlow JD, Morrey ME, Moran SL, et al. Proximal Humerus Reconstruction for Bone Sarcomas: A Critical Analysis. JBJS Rev. 2024;12(3):e23.00217. 10.2106/JBJS.RVW.23.00217. [DOI] [PubMed]
- 54.Sirveaux F. Reconstruction techniques after proximal humerus tumour resection. Orthop Traumatol Surg Res. 2019;105(1S):S153–64. 10.1016/j.otsr.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 55.Boileau P, Morin-Salvo N, Bessiere C, Chelli M, Gauci MO, Lemmex DB. Bony increased-offset-reverse shoulder arthroplasty: 5 to 10 years’ follow-up. J Shoulder Elb Surg. 2020;29(10):2111–22. 10.1016/j.jse.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Stephenson DR, Oh JH, McGarry MH, Hatch GF III, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652–8. 10.1016/j.jse.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 57.Gulotta LV, Choi D, Marinello P, Knutson Z, Lipman J, Wright T, et al. Humeral component retroversion in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elb Surg. 2012;21(9):1121–7. 10.1016/j.jse.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 58.Goldman LH, Morse LJ, O’Donnell RJ, Wustrack RL. How often does spindle failure occur in compressive osseointegration endoprostheses for oncologic reconstruction? Clin Orthop Relat Res. 2016;474(7):1714–23. 10.1007/s11999-016-4839-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prophylactic Antibiotic Regimens in Tumor, Surgery I, Ghert M, Schneider P, Guyatt G, Thabane L, Velez R, et al. Comparison of prophylactic intravenous antibiotic regimens after endoprosthetic reconstruction for lower extremity bone tumors: a randomized clinical trial. JAMA Oncol. 2022;8(3):345–53. 10.1001/jamaoncol.2021.6628. [DOI] [PMC free article] [PubMed]
- 60.Cipriano C, Griffin AM, Ferguson PC, Wunder JS. Developing an evidence-based Followup schedule for bone sarcomas based on local recurrence and metastatic progression. Clin Orthop Relat Res. 2017;475(3):830–8. 10.1007/s11999-016-4941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.