Abstract
Introduction
Acupuncture is widely used for metabolic-associated fatty liver disease (MAFLD) treatment; however, the clinical efficacy has not been confirmed due to the lack of high-level evidence-based clinical practice. The purpose of this study is to design a research protocol that will be used to determine the efficacy of acupuncture versus sham acupuncture (SHA) for MAFLD treatment.
Methods and analysis
This will be a multicentre, randomised and sham-controlled trial. Ninety-eight participants with MAFLD will be enrolled in this trial. Participants will be randomly assigned in a 1:1 ratio to receive acupuncture or SHA for 12 weeks. The primary outcome is the rate of patients with a 30% relative decline in liver fat after 12 weeks of treatment in MRI-proton density fat fraction (MRI-PDFF), which will be obtained by quantitative chemical shift imaging such as the multipoint Dixon method at 0, 12 and 24 weeks. Secondary outcomes include the changes in the relative liver fat content measured by MRI-PDFF, magnetic resonance elastography, liver function, lipid metabolism, homeostatic model assessment for insulin resistance (HOMA-IR) and serum high sensitivity C reactive protein, which will be obtained at 0, 6, 12 and 24 weeks. Body measurement indicators (body mass index, waist circumference, hip circumference and waist-to-hip ratio) will be obtained at 0, 3, 6, 9, 12 and 24 weeks. The alteration in the gut microbiota composition and its metabolism will be assessed by 16S ribosomal RNA sequencing and liquid chromatography-mass spectrometry at 0 and 12 weeks.
Ethics and dissemination
This study protocol has been approved by the ethics committee of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (2023-1347-114-01). The results of this study will be published in a peer-reviewed journal and presented at academic conferences.
Trial registration number
ChiCTR2300075701.
Keywords: complementary medicine, hepatology, randomized controlled trial
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This will be the first multicentre study to assess the effects of acupuncture in treating metabolic-associated fatty liver disease (MAFLD) based on MRI-proton density fat fraction.
Gut microbiota is identified for exploring the efficacy of acupuncture in the treatment of MAFLD.
Acupuncturists will insert needles into the patients’ bodies, so acupuncturists can not be blind to the treatment.
Introduction
Fat accumulation in the liver is a prevalent disorder affecting up to 40% of the global population.1 Metabolic-associated fatty liver disease (MAFLD) is characterised by fat accumulation with varying degrees of inflammation and fibrosis, becoming a significant global health.2 MAFLD is more prevalent in Western (20%–30%) than in Eastern countries (10%–20%) and often leads to cardiovascular diseases, such as hypertension, atherosclerosis and cardiac diseases.3 4 Additionally, patients with MAFLD with advanced fibrosis and cirrhosis are at increased risk of hepatocellular carcinoma.5
The pathogenesis of MAFLD remains unclear. Previous studies have revealed multiple mechanisms involved in MAFLD, including hepatic steatosis, glycolipid metabolism, insulin resistance (IR), gut–liver axis unbalance and metabolites of intestinal microbiota.6,8 Therefore, effective treatments targeting multiple pathways need to be explored. Although dietary restriction and exercise are generally recommended for MAFLD, patients often find it difficult to persist.9 New treatment strategies for MAFLD are necessary.
Acupuncture, an alternative therapy of traditional Chinese medicine (TCM), has been practised for thousands of years in China and other Asian countries, such as Japan and Korea. Recent research has demonstrated that acupuncture may effectively mitigate hepatic steatosis, improve glycolipid metabolism and IR and regulate intestinal microbiota composition, suggesting that acupuncture is a potentially effective therapy for MAFLD.10,12 Many studies have focused on non-pharmacologic therapies in clinical practice, with acupuncture increasingly being used as a treatment for MAFLD.13 However, there are no high-quality clinical studies to determine the efficacy of acupuncture for the MAFLD treatment. Therefore, we design a multicentre, randomised and controlled trial that will be used to evaluate the effects of acupuncture versus sham acupuncture (SHA) in treating MAFLD.
Methods/design
Study design
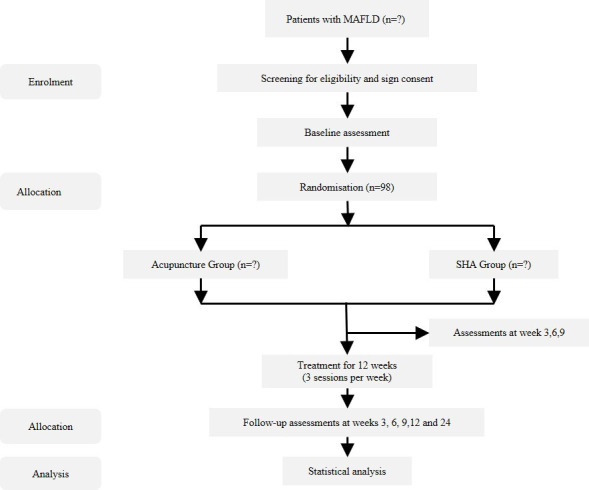
The multicentre, randomised and sham-controlled trial will be conducted at three hospitals in Shanghai: (1) Shuguang Hospital Affiliated to Shanghai University of TCM, (2) Longhua Hospital Affiliated to Shanghai University of TCM and (3) Yueyang Hospital Affiliated to Shanghai University of TCM. The study protocol has been approved by the ethics committees of all hospitals. The study will be conducted according to the Declaration of Helsinki and reported following the Standard Protocol Items: Recommendations for Interventional Trials guidelines (online supplemental additional file 1).14 The flow diagram of the clinical trial approach is shown in figure 1.
Figure 1. Flow diagram of the trial. MAFLD, metabolic-associated fatty liver disease; SHA, sham acupuncture.
Patient and public involvement
Patients and/or the public will not be involved in the design, practice, reporting, or dissemination of this research.
Population and recruitment
MAFLD is diagnosed according to The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of MAFLD.15 Once informed consent is obtained, randomisation will be performed (onlinesupplemental materials 1 2). The schedule of enrolment, intervention and assessments is shown in table 1.
Table 1. The schedule of enrolment, intervention and assessments.
| Study period | |||||||
| Enrolment | Allocation | Post allocation | Closeout | ||||
| Time point (week) | −2 | 0 | 3 | 6 | 9 | 12 | 24 |
| Enrolment | |||||||
| Eligibility screen | X | ||||||
| Informed consent | X | ||||||
| Randomisation | X | ||||||
| Intervention | |||||||
| Acupuncture | X | X | X | X | |||
| Sham acupuncture | X | X | X | X | |||
| Assessments | |||||||
| General information | X | X | |||||
| MRI-PDFF | X | X | X | ||||
| Liver function | X | X | X | X | |||
| Lipids metabolism | X | X | X | X | |||
| HOMA-IR | X | X | X | X | |||
| hs-CRP | X | X | X | X | |||
| Adipocytokines | X | X | X | X | |||
| BMI, WC, HC, WHR | X | X | X | X | X | X | |
| MRE | X | X | X | ||||
| Gut microbiota | X | X | |||||
| Adverse events | X | X | X | X | X | X | |
BMIbody mass indexHChip circumferenceHOMA-IRhomeostatic model assessment for insulin resistancehs-CPRhigh sensitivity C reactive proteinMREmagnetic resonance elastographyMRI-PDFFMRI-proton density fat fractionWCwaist circumferenceWHRwaist-to-hip ratio
Inclusion criteria
Aged 18–70 years (either sex).
Confirmation of MAFLD according to guidelines.15
Stagnation of dampness and turbidity, accumulation of dampness and heat, and mutual accumulation of phlegm and blood stasis confirmed by TCM syndrome classification.16
Body mass index (BMI)≥23 kg/m2.
MAFLD with MRI-proton density fat fraction (MRI-PDFF)≥11%.
Written informed consent.
Exclusion criteria
Hepatic and biliary diseases induced by other causes, including but not limited to hepatitis B or C virus infection, alcohol-associated liver disease, drug-induced liver disease, autoimmune hepatitis, cirrhosis, primary sclerosing cholangitis, Wilson’s disease, α-1 antitrypsin deficiency and liver cancer (or a family history of liver cancer).
Severe liver function injury, defined as alanine transaminase (ALT)≥2.5 times the upper limit of normal.17,19
Clinical evidence of the presence of cirrhosis or decompensation of liver function.
Severe renal insufficiency.
Primary diseases such as cardiovascular, cerebrovascular, urinary and hematopoietic systems, malignant tumours, other serious comorbidities, or psychiatric disorders.
Combined with type 1 diabetes or uncontrolled type 2 diabetes.
Combined with thyroid disease, including hyperthyroidism, hypothyroidism and subclinical hypothyroidism.
Contraindications to MRI, including but not limited to severe claustrophobia, inner ear implants, pacemakers or other implanted rhythm management equipment, intracranial aneurysm clips incompatible with MRI, any other metal, non-MRI compatible implantation equipment (such as insulin pump, medullary joint replacement), medical history of orbital metal fragments that have not been removed and weight or waist circumference (WC) that exceeds the scanner function.
Those who have received acupuncture treatment before enrolment within at least 1 year.
Pregnant and lactating women, as well as women who are at risk of pregnancy refuse to maintain contraceptive measures recognised by the researchers.
Participated in other clinical trials in the past 3 months.
Those who are unable to follow medical advice for lifestyle interventions.
Other situations that are not suitable for participation in this study were identified by the researchers.
Randomisation and allocation concealment
Eligible patients will be randomly assigned to the acupuncture group or SHA group in a 1:1 ratio. A random allocation sequence number will be generated using the blockrand package of R V.4.1.3 by the relevant statisticians. Each random number will be written on letter paper and sealed in an opaque envelope. Participants will draw an envelope to obtain their random number, which will then be recorded in a case report form (CRF).
Masking
Patients, outcome assessors and statisticians who perform the statistical analyses will be blind to group assignment. To ensure the implementation of blinding, patients in different groups will be arranged to be treated and followed up in separate rooms. Throughout the study, patients from different groups will not meet or converse with each other. On the completion of the study, questionnaires are distributed to participants, asking them whether they are aware of their assigned group and the reasons for their beliefs.20
Sample size
Sample size was calculated based on the formula:
where represents the number of participants in each group; represents the overall rate of the test group (55%), represents the overall rate value of the control group (25%) and represents the difference between the two groups (30%).21 Superiority margin is 5%.22 Based on a previous report with the p value of 0.05 and power of 80%, the expected difference in MRI-PDFF is 30%.23 24 Through calculation, the sample size in each group is 45. The sample size is inflated to allow for a 10% dropout rate over the course of the study, and finally, the calculated sample size in each group is approximately 49.
Interventions
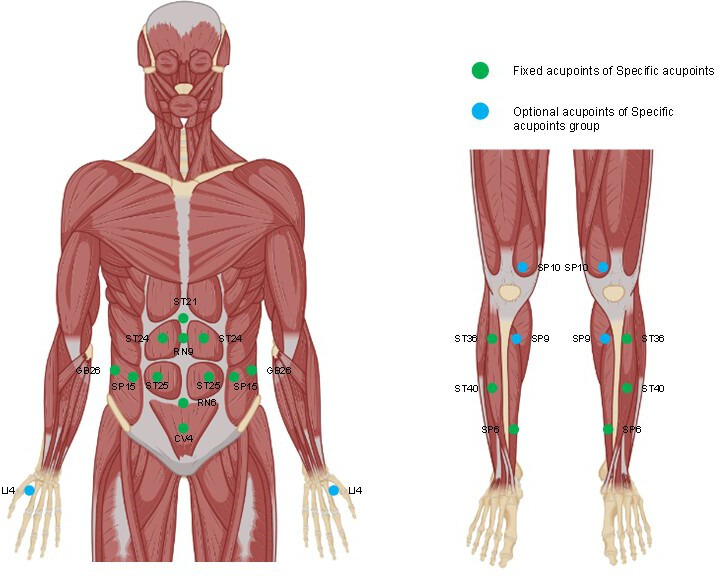
Following a 2-week washout period, the subjects will receive 30 min of acupuncture treatment three times weekly for 12 weeks. The practice of conducting multiple treatments within a single day will be expressly prohibited. The treatment protocol has been mastered by all acupuncturists. The acupuncture point locations are shown in figure 2.
Figure 2. Locations of acupoints.
Acupuncture group
The fixed acupoints include Zhongwan (RN12), Shuifen (RN9), Guanyuan (CV4), Qihai (RN6), Huaroumen (ST24), Tianshu (ST25), Daheng (SP15), Daimai (GB26), Fenglong (ST40), Zusanli (ST36) and Sanyinjiao (SP6), which are used to treat patients with MAFLD in published papers.25 In addition, according to TCM syndrome differentiation, the syndrome of stagnation of dampness and turbidity will be treated with Hegu (LI4), the syndrome of accumulation of dampness and heat will be treated with Yinlingquan (SP9) and the syndrome of mutual accumulation of phlegm and blood stasis will be treated with Xuehai (SP10).16 26 After sterilisation, the 0.25 mm×60 mm needles (Hwato, Suzhou, China) will be inserted vertically into acupoint sites and then the manipulations of twirling will be practised. The location of acupoints is shown in table 2.
Table 2. The location of the acupoints.
| Acupoints | Location |
| Fixed acupoints | |
| Zhongwan (RN12) | On the anterior midline, 4 cun above the umbilicus. |
| Shuifen (RN9) | On the anterior midline, 1 cun above the umbilicus. |
| Guanyuan (CV4) | On the anterior midline, 3 cun below the umbilicus. |
| Qihai (RN6) | On the anterior midline, 1.5 cun below the umbilicus. |
| Huaroumen (ST24) | On the anterior midline, 1 cun above the umbilicus and 2 cun lateral to the anterior midline. |
| Tianshu (ST25) | On the same level of the umbilicus and 2 cun lateral to the anterior midline. |
| Daheng (SP15) | On the same level of the umbilicus and 4 cun lateral to the anterior midline. |
| Daimai (GB26) | At the intersection of the perpendicular line below the free edge of the 11th rib and the horizontal line of the umbilical cord. |
| Fenglong (ST40) | One finger-breadth lateral to Tiaokou and at the midpoint of the line joining Dubi and the tip of the external malleolus. |
| Zusanli (ST36) | 3 cun directly below Dubi and 1 finger-breadth lateral to the anterior border of the tibia. |
| Sanyinjiao (SP6) | Posterior to the mesial border of the tibia and 3 cun above the tip of the medial malleolus. |
| Matching acupoints | |
| Hegu (LI4) | Between the first and second metacarpal bones and in the midpoint of the radial side of the second metacarpal bone; or, the transverse crease of interphalangeal joint of one thumb are placed on the stretched hukou between the thumb and index finger of the other hand, and the acupoint is where the tip of the thumb touches. |
| Yinlingquan (SP9) | Posteroinferior to the medial condyle of the tibia. |
| Xuehai (SP10) | 2 cun above the upper border of the medial patella; or the acupoint is where the tip of the thumb when the doctor puts his right palm on the left knee of the patient and with the centre of the palm pointing to the centre of patella of patient and the patient flexes his knee joint and makes it to be a right angle (right Xuehai is measured by the left hand) |
SHA group
In this group, the SHA needles (0.25 mm × 60 mm) featuring flat tips (Hwato, Suzhou, China) will be inserted into the same acupoints as the acupuncture group.27 28
Diet and exercise
Based on the guidance for prescribing exercise reported in Med Sci Sports Exerc 2011, subjects are recommended to keep 30 min of moderate-intensity exercise per day, like jogging, swimming, Tai Chi and cycling.29 In addition, a hypocaloric diet (500–1000 kcal deficit) is recommended for all subjects, such as 100 g of cake, 200 g of fried chicken, 400 g of rice, 120 g of pork and any vegetables.15 During the treatment, all subjects are asked to complete the questionnaire concerning the dietary pattern. This questionnaire encompasses a prescribed low-fat and low-carbohydrate plan. Response options include none, <50% of the time or ≥50% of the time. Additionally, participants will be also asked to report their levels of daily physical activity. Specifically, they will be asked about the frequency of engaging in exercise sessions lasting at least 30 min during the study. The response alternatives include exercising less than once a week, engaging in physical activity once to two times per week or participating in such activities three times a week or more, as shown in table 3.
Table 3. Diet and exercise record table.
| Name | ID | Date |
| Have you followed dietary recommendations this week, such as a low-fat and low-carbohydrate diet? |
|
|
| Have you followed exercise recommendations this week, such as 30 min/day of jogging for ≥5 days/week? |
|
Outcomes
Primary outcome
The primary outcome is the rate of patients with a 30% relative decline in liver fat assessed by MRI-PDFF after 12 weeks of treatment.23 24
Secondary outcomes
The secondary outcomes include the changes as follows: (1) liver fat content measured by MRI-PDFF at 12 weeks, (2) the degree of liver fibrosis assessed by magnetic resonance elastography (MRE), (3) hepatic enzymes including ALT, aspartate transaminase and γ-glutamyl transferase levels in serum, (4) indicator of lipid profiles (triglycerides, total cholesterol, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol, (5) serum high sensitivity C reactive protein levels, (6) serum adipocytokines including leptin, adiponectin and resistin, (7) anthropometric measurements including BMI, WC, hip circumference and waist-to-hip ratio and (8) HOMA-IR.
Faecal microbiota assessment
The count and composition of operational taxonomic units within the gut microbiota will be tested by 16S ribosomal RNA sequencing. We will also measure the α-diversity indices, including Sobs, Chao1, Ace, Shannon, Simpson and Coverage, to gauge the diversity within the microbial population. In order to probe the differences in community composition, β-diversity analyses will be conducted using the unweighted and weighted UniFrac metrics. In addition, the untargeted metabolomics analysis will be conducted by using liquid chromatography-mass spectrometry to investigate the impact of acupuncture on gut microbiota metabolism.
Statistical analysis
The data will be analysed by using SPSS (V.22.0.0). Descriptive analyses will be performed for all baseline variables. Continuous variables under an assumption of normality are described as mean±SD, and those with a non-normal distribution are described as medians and quartiles (Q1; Q3). Categorical variables are represented as absolute values with percentages.
Baseline demographic and clinical data will be analysed using the t-test (normally distributed) or Mann-Whitney U test (non-normally distributed) for continuous variables, or the χ2 test for categorical variables.
Intention-to-treat set will be used for the analysis of the controlled trial. The χ2 test will be used to compare the differences in the primary outcome between the acupuncture group and the SHA group. In instances of missing or inconsistent data, the last observation carried forward method or multiple imputation method will be done. Differences in the secondary outcomes between the acupuncture group and the SHA group will be assessed by using the linear mixed model.
In the per-protocol set, a sensitivity analysis will be conducted. All statistical analyses will be performed using SAS V.9.4 and R V.4.1.2 (https://www.r-project.org/).
Permutational multivariate analysis of variance will be used to analyse the correlation between gut microbiome and clinical characteristics. This analysis will be done on the Bray-Curtis dissimilarity.30 The non-parametric Mann-Whitney U test will be performed to analyse the alteration of gut microbiota’s relative abundance before and after treatment. Pearson’s correlation analysis will be done between changes in metabolites originating from the gut microbiota and enhancements in clinical variables.
In the aforementioned statistical analyses, a p value<0.05 is regarded as indicative of statistical significance.
Adverse events
During the trial, all adverse events will be observed by the investigator and reported by the subjects. The possible treatment-related adverse events may include subcutaneous haematoma, postneedling discomfort, pruritus at the needle insertion sites and sensations of dizziness, which will be analysed using a χ2 test or Fisher’s exact test.31 32
Data management
All data will be initially recorded on CRF and inputted into the data management system of this study by investigators. Pertinent characteristics of all patients will be recorded as an anonymised version, such as names, ID card numbers and telephone numbers.
Records containing personal information on patients will be held securely for a minimum of 5 years. In each clinical centre, the data are evaluated by controllers. Controllers will monitor the progress of the trial and evaluate the relevant adverse events, which will be independent of the investigators.
Quality control
The design of this trial has been evaluated and refined by experts specialising in acupuncture, gastroenterology, statistics and methodology. All modifications made to this trial will be reported to the ethics committee of Shuguang Hospital Affiliated to Shanghai University of TCM. Any alteration made to our database will be recorded on CRF.
Ethics and dissemination
This trial was registered in the Chinese Clinical Trial Registry (ChiCTR-2300075701). Furthermore, the study protocol has been approved by the ethics committee of Shuguang Hospital Affiliated to Shanghai University of TCM (No. 2023-1347-114-01). Patients who meet the inclusion criteria in 3 clinical centres will be enrolled after obtaining written informed consent from patients. If the protocol amendments are required, all pertinent materials will be reported to the ethics committee.
The independent data monitoring committee will be used to review the progression and the collected data during the study in Shuguang Hospital Affiliated to Shanghai University of TCM. The committee holds the authority to initiate necessary modifications or even terminate the trial.
Study outcomes will be disseminated via peer-reviewed journals and presented at academic conferences.
Discussion
Previous research has indicated that acupuncture may have benefits in reducing liver fat content among individuals with MAFLD.21 25 However, multicentre randomised controlled clinical trials are essential to properly evaluate the role of acupuncture in treating MAFLD. Therefore, our study will provide high-quality evidence to assess the clinical effectiveness of acupuncture for MAFLD.
Blunt-tipped placebo needles have been used in previous studies to identify the efficacy of acupuncture in treating MAFLD.20 31 The selection of acupoints is considered one of the most pivotal factors influencing the effectiveness of acupuncture.20
Although liver biopsy is the gold standard for diagnosing MAFLD, its invasive nature limits its use in clinical studies. In December 2018, the Food and Drug Administration (FDA) issued guidelines suggesting the adoption of MRI-PDFF for assessing therapeutic efficacy in early-phase clinical trials for non-alcoholic steatohepatitis.2 3 33 Research shows that a relative decrease in MRI-PDFF≥30% is significantly correlated with histological improvement in MAFLD, making it a useful criterion for evaluating clinical effects.33
There is a growing body of evidence that the gut microbiota are involved in the onset and progression of MAFLD.34,36 Alterations in gut microbiota can disrupt beneficial microbial populations, affecting the integrity of the intestinal mucosal barrier.37 A high-fat diet can alter gut microbiota, leading to increased intrahepatic lipids, liver enzymes and histologically confirmed steatosis in patients with MAFLD.38
Metabolites originating from the gut microbiota can modulate the intestinal morphology and immune response, changing with the severity and fibrosis stage of MAFLD. Perturbation in amino acid metabolism, especially aromatic amino acids, glutamate–serine–glycine index and branched-chain amino acids, has been implicated in the pathogenesis of MAFLD and MASH.39 40 To further explore the potential mechanism of acupuncture in treating MAFLD, faecal samples will be collected before and after treatment to analyse gut microbiota composition. These alterations may shed light on the effective mechanisms of acupuncture.
However, there are some limitations in this study. First, only the obese patients with MAFLD are enrolled in this study, therefore we cannot provide evidence of acupuncture’s effectiveness for treating lean patients with MAFLD. Second, as liver biopsy is not used as a therapeutic indicator, we cannot compare the sensitivity and specificity of MRE and liver biopsy in evaluating the clinical effects of acupuncture. Finally, the treatment course is short, and future studies should explore the long-term clinical efficacy.
Trial status
At the time of manuscript submission, recruitment work for the trial is ongoing. The protocol version is MAFLD V.8.16, 16 August 2023. The recruitment of this study began on 15 September 2023 and will be ongoing until 31 January 2025.
supplementary material
Acknowledgements
The authors greatly appreciated Dr Burhan Yokus (NIAAA, NIH) for critical reading and editing the manuscript.
Footnotes
Funding: This study was supported by National Center for Inheritance and Innovation of Traditional Chinese Medicine, Pudong Famous Traditional Chinese Medicine Training Plan of Shanghai (PWRzm2020-02), High level key disciplines of Medicine of National Administration of Traditional Chinese Medicine (ZYYzDXK-2023060).
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-081293).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Lihong Fu, Email: 18083883901@163.com.
Lingying Huang, Email: hly320@126.com.
Yueqiu Gao, Email: gaoyueqiu@hotmail.com.
Wanchun Zhu, Email: 1397804774@qq.com.
Yu Cui, Email: cyyyyy0627@163.com.
Shihao Wang, Email: 1569557054@qq.com.
Meihua Yan, Email: yanhm2011@126.com.
Jing Li, Email: 1971921250@qq.com.
Junyi Duan, Email: duanjunyicn@163.com.
Jielu Pan, Email: yearlca@126.com.
Man Li, Email: liman121000@shutcm.edu.cn.
References
- 1.Nascimbeni F, Pais R, Bellentani S, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859–71. doi: 10.1016/j.jhep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 2.Badmus OO, Hillhouse SA, Anderson CD, et al. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways. Clin Sci. 2022;136:1347–66. doi: 10.1042/CS20220572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, He W, Tsai P-J, et al. Mutual interaction between endoplasmic reticulum and mitochondria in nonalcoholic fatty liver disease. Lipids Health Dis. 2020;19:72.:72. doi: 10.1186/s12944-020-01210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoo TK, Lee MY, Kim SH, et al. Comparison of cardiovascular mortality between MAFLD and NAFLD: A cohort study. Nutr Metab Cardiovasc Dis. 2023;33:947–55. doi: 10.1016/j.numecd.2023.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Song BG, Choi SC, Goh MJ, et al. Metabolic dysfunction-associated fatty liver disease and the risk of hepatocellular carcinoma. JHEP Rep . 2023;5:100810. doi: 10.1016/j.jhepr.2023.100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballestri S, Nascimbeni F, Romagnoli D, et al. The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol Res. 2016;46:1074–87. doi: 10.1111/hepr.12656. [DOI] [PubMed] [Google Scholar]
- 7.Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Sharpton SR, Ajmera V, Loomba R. Emerging role of the gut microbiome in nonalcoholic fatty liver disease: from composition to function. CLIN GASTROENTEROL Hepatol. 2019;17:296–306. doi: 10.1016/j.cgh.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semmler G, Datz C, Reiberger T, et al. Diet and exercise in NAFLD/NASH: Beyond the obvious. Liver Int. 2021;41:2249–68. doi: 10.1111/liv.15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong C, Zhang C-R, Xue B-Y, et al. Electroacupuncture combined with lifestyle control on obese nonalcoholic fatty liver disease: a randomized controlled trial. Chin Acupunct Moxibust. 2020;40:129–34. doi: 10.13703/j.0255-2930.20190201-k00034. [DOI] [PubMed] [Google Scholar]
- 11.Han J, Guo X, Meng X-J, et al. Acupuncture improved lipid metabolism by regulating intestinal absorption in mice. World J Gastroenterol. 2020;26:5118–29. doi: 10.3748/wjg.v26.i34.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Wang Q, Liang C, et al. Acupuncture improved hepatic steatosis in HFD-induced NAFLD rats by regulating intestinal microbiota. Front Microbiol. 2023;14:1131092. doi: 10.3389/fmicb.2023.1131092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su D, Li L. Trends in the use of complementary and alternative medicine in the United States: 2002-2007. J Health Care Poor Underserved. 2011;22:296–310. doi: 10.1353/hpu.2011.0002. [DOI] [PubMed] [Google Scholar]
- 14.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eslam M, Sarin SK, Wong VW-S, et al. The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889–919. doi: 10.1007/s12072-020-10094-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhao W, Xv E, Wang X, et al. Traditional Chinese medicine guidelines for the diagnosis and treatment of non-alcoholic fatty liver disease (in Chinese) J Clin. 39:1041–8. doi: 10.3969/j.issn.1001-5256.2003.05.007. n.d. [DOI] [Google Scholar]
- 17.Jasiewicz M, Siedlaczek M, Kasprzak M, et al. Elevated serum transaminases in patients with acute coronary syndromes: Do we need a revision of exclusion criteria for clinical trials? Cardiol J. 2023;30:747–52. doi: 10.5603/CJ.a2021.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebovitz HE, Kreider M, Freed MI. Evaluation of liver function in type 2 diabetic patients during clinical trials: evidence that rosiglitazone does not cause hepatic dysfunction. Diabetes Care. 2002;25:815–21. doi: 10.2337/diacare.25.5.815. [DOI] [PubMed] [Google Scholar]
- 19.Lee WM, Larrey D, Olsson R, et al. Hepatic findings in long-term clinical trials of ximelagatran. Drug Saf. 2005;28:351–70. doi: 10.2165/00002018-200528040-00006. [DOI] [PubMed] [Google Scholar]
- 20.Qi L-Y, Yang J-W, Yan S-Y, et al. Acupuncture for the treatment of diarrhea-predominant irritable bowel syndrome: A pilot randomized clinical trial. JAMA Netw Open. 2022;5:e2248817. doi: 10.1001/jamanetworkopen.2022.48817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J, Wang Q, Zhao X, et al. Electro-acupuncture reduced steatosis on MRI-PDFF in patients with non-alcoholic steatohepatitis: a randomized controlled pilot clinical trial. Chin Med. 2023;18:19. doi: 10.1186/s13020-023-00724-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu YX, Yao C, Chen F, et al. Statistical methods for clinical non-inferiority/equivalence evaluation (in Chinese) Chin J Clin Pharmacol Ther. 2000;5:344–8. [Google Scholar]
- 23.Jinato T, Chayanupatkul M, Dissayabutra T, et al. Litchi-derived polyphenol alleviates liver steatosis and gut dysbiosis in patients with non-alcoholic fatty liver disease: A randomized double-blinded, placebo-controlled study. Nutrients. 2022;14:2921. doi: 10.3390/nu14142921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2016;9:692–701. doi: 10.1177/1756283X16656735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen P, Zhong X, Dai Y, et al. The efficacy and safety of acupuncture in nonalcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Medicine (Balt) 2021;100:e27050. doi: 10.1097/MD.0000000000027050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao W, Xu E, Wang X, et al. Guidelines for traditional Chinese medicine diagnosis and treatment of non alcoholic fatty hepatitis (in Chinese) Chin J Integr Tradit West Med Liver Dis. 32:1059–62. n.d. [Google Scholar]
- 27.Park J, White A, Lee H, et al. Development of a New Sham Needle. Acupunct Med. 1999;17:110–2. doi: 10.1136/aim.17.2.110. [DOI] [Google Scholar]
- 28.Liang Z-H, Xie C-C, Li Z-P, et al. Deqi sensation in placebo acupuncture: A crossover study on Chinese medicine students. Evid Based Complement Alternat Med. 2013;2013:620671.:620671. doi: 10.1155/2013/620671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 30.Anderson MJ. A new method for non‐parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x. [DOI] [Google Scholar]
- 31.Bao C, Wu L, Wang D, et al. Acupuncture improves the symptoms, intestinal microbiota, and inflammation of patients with mild to moderate Crohn’s disease: A randomized controlled trial. E Clin Med. 2022;45:101300. doi: 10.1016/j.eclinm.2022.101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi L-Y, Yang J-W, Yan S-Y. Acupuncture for the treatment of diarrhea-predominant irritable bowel syndrome: A pilot randomized clinical trial. JAMA Netw Open. 2022;5:e2248817. doi: 10.1001/jamanetworkopen.2022.48817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stine JG, Munaganuru N, Barnard A, et al. Change in MRI-PDFF and histologic response in patients with nonalcoholic steatohepatitis: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:2274–83. doi: 10.1016/j.cgh.2020.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung C, Rivera L, Furness JB, et al. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–25. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 35.Tripathi A, Debelius J, Brenner DA, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, et al. Nonalcoholic fatty liver disease: Modulating gut microbiota to improve severity? Gastroenterology. 2020;158:1881–98. doi: 10.1053/j.gastro.2020.01.049. [DOI] [PubMed] [Google Scholar]
- 37.Ruuskanen MO, Åberg F, Männistö V, et al. Links between gut microbiome composition and fatty liver disease in a large population sample. Gut Microbes. 2021;13:1–22.:1888673. doi: 10.1080/19490976.2021.1888673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu C-C, Ching Y-H, Li Y-P, et al. Nonalcoholic fatty liver disease is exacerbated in high-fat diet-fed gnotobiotic mice by colonization with the gut microbiota from patients with nonalcoholic steatohepatitis. Nutrients. 2017;9:1220. doi: 10.3390/nu9111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaggini M, Carli F, Rosso C, et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology. 2018;67:145–58. doi: 10.1002/hep.29465. [DOI] [PubMed] [Google Scholar]
- 40.Hoyles L, Fernández-Real J-M, Federici M, et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med. 2018;24:1070–80. doi: 10.1038/s41591-018-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


