Abstract
Verruca vulgaris is a cutaneous infection predominantly caused by human papillomavirus (HPV) type 1, 2, and 4. In immunocompromised individuals infected with human immunodeficiency virus (HIV) infection, HPV leads to a higher prevalence of infections and also has a greater likelihood of being infected with atypical types such as genital-associated HPV in extragenital sites. This case report describes a 48-year-old male patient who presented with skin-colored verrucous papules on the hands and feet, with no evidence of genital lesions. Polymerase Chain Reaction (PCR) genotyping identified the presence of HPV types 4, 6, and 16 as an etiology of verruca vulgaris, low-risk HPV with genital-associated lesions, and high-risk HPV. This atypical result may suggest a failure of the immune defense system of the body. Therefore, accurately identifying HPV types through PCR testing in immunocompromised patients is essential for appropriate clinical management and monitoring.
Keywords: verruca vulgaris, human papillomavirus type 6, human immunodeficiency virus, genotyping, polymerase chain reaction
Introduction
Verruca vulgaris or common wart is a viral skin infection caused by human papillomavirus (HPV),1,2 a member of the Papillomaviridae family with double-stranded deoxyribonucleic acid (DNA).3 Clinical manifestations of HPV can occur on mucosal surfaces and skin throughout the body.4,5 HPV skin infections are estimated to affect 10% of the global population,1 occurring across all age groups. The infections are more commonly observed in children and adults, with an incidence rate and prevalence of 30% and 0.84–12.9%, respectively.6,7 There are over 150 hPV subtypes, each with a tendency to infect specific anatomical sites.1 However, in immunocompromised individuals, cutaneous wart lesions can be attributed to HPV types typically associated with genital infections.8,9
Among the various types of cutaneous warts, verruca vulgaris is the most prevalent, predominantly caused by HPV types 1, 2, and 4.1
HPV types causing benign lesions and anogenital warts are classified as low-risk, while those associated with cancer and malignant lesions are considered high-risk.6,10 Low-risk HPV types such as 6 and 11 are associated with anogenital warts with low potential for malignancy. Meanwhile, high-risk types, such as 16 and 18, are closely connected to significantly increased risk of malignancy.11 Lesions associated with HPV infection include cervical, vaginal, vulvar, penile,12,13 anal cancer,12,14 and oropharyngeal cancers, which occur in persistent high-risk HPV infections, worsen from precancerous and cancerous.14,15 HPV causes an estimated 630,000 cancers worldwide, accounting for about 5%.14 In 2019, the virus led to an estimated 620.000 and 70.000 cancer cases in females and males, respectively.15 Warts can significantly impair the quality of life of patients due to physical discomfort and cosmetic disfigurement.16 Extragenital warts caused by HPV type 6 are uncommon, with the etiology not well understood.17 The prevalence has not been extensively studied but a case series17 and multiple case reports have explored the topic.17–19 This occurrence of extragenital warts caused by genital-associated, and high-risk HPV is more commonly found in an immunocompromised individual including human immunodeficiency virus (HIV) infection.9,20 Other viruses, such as herpes simplex and Epstein-Barr virus, as well as various bacterial species, frequently interact with HPV, potentially influencing the replication, persistence, and progression to cancer.21 The evidence shows that HPV6 and HPV11 may contribute to the development of malignant lesions, with reported frequencies of single or dual HPV6/11 infections varying between 0–5.5% and 0–87.5% for penile and laryngeal cancers, respectively.22 Previous research have reported peculiar characteristics of the immune response to HPV type 6, including the transfer of antibodies from the mother to the child.23 Therefore, this report aimed to describe a case of verruca vulgaris in an HIV patient with Polymerase Chain Reaction (PCR) genotyping results.
Case Report
A 48-year-old male presented at Venereology Clinic Doctor Hasan Sadikin General Hospital (RSHS) with chief complaint of skin-colored papules with rough surfaces occasionally pruritic but non-painful on both hands and feet. These lesions had been present for approximately three years, with the initial beginning as two pinhead-sized, mildly pruritic, non-painful papules on the dorsal right hand. Over time, the lesions extended to both hands and feet, causing discomfort and social embarrassment. The patient was treated at a local clinic, where a diagnosis of warts was made, and a topical treatment was prescribed. After three months, there was no noticeable improvement. Subsequently, the patient self-administered Callusol® (containing salicylic acid, lactic acid, and polidocanol) and Benoson® (containing betamethasone) for six months with no improvement.
The patient works as a construction worker and frequently experiences minor injuries to the hands and feet. A similar condition was reported in a coworker but denied any family history of similar lesions. Good personal hygiene was practiced, avoiding shared towels and wearing footwear in public facilities. The patient had a significant medical history of HIV stage 4, diagnosed in May 2023, and was on antiretroviral therapy. A history of intravenous drug use was also reported, and HPV vaccine was not received. The patient has tattoos, smokes, and occasionally consumes alcohol. There are no systemic symptoms or drug allergies reported and screening for pulmonary tuberculosis was negative.
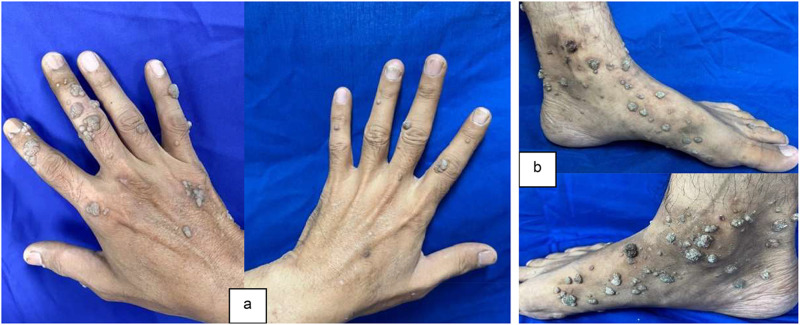
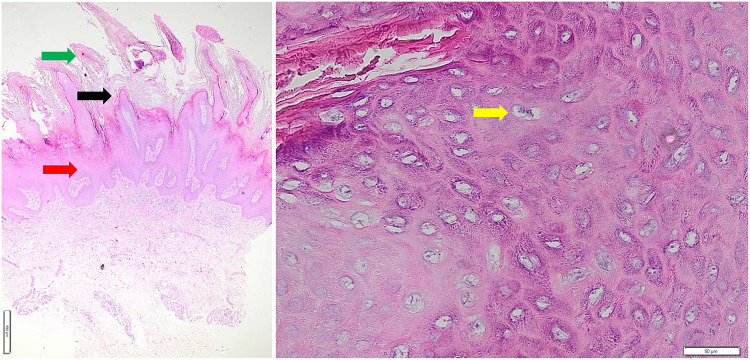
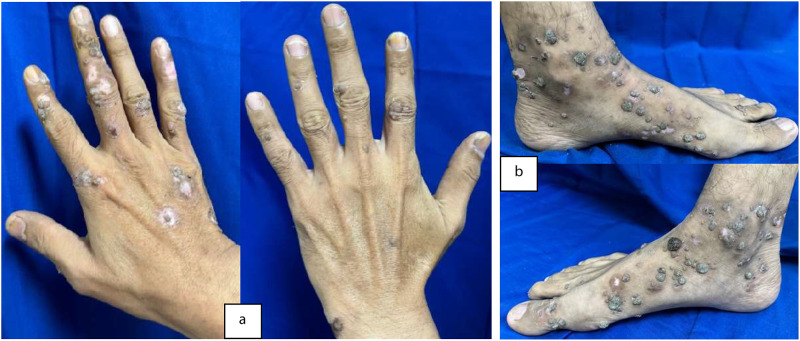
Physical examination showed normal vital signs and dermatological status reported multiple lesions with a regional distribution on both hands and feet. The lesions were partially discrete and confluent, with irregular shapes. The smallest lesion measures 0.1 cm x 0.1 cm x 0.1 cm to 3 cm x 2 cm x 0.1 cm. The lesions had well-defined borders raised from the skin surface, dry, and presented as verrucous papules and plaques (Figure 1). Meanwhile, the dermoscopic examination of the verrucous papules showed dotted vessels (Figure 2). Histopathological examination of the verrucous lesions on the feet showed acanthosis, epidermal hyperplasia, papillomatosis, orthokeratosis, and koilocytosis, with no evidence of malignancy (Figure 3). The CD4 level at the time was 106/Ul and the patient was initially subjected to cryotherapy at two-week intervals for three months. However, the patient reported intolerable side effects, including pain and blister formation. An alternative treatment option was requested, and the use of 80% trichloroacetic acid (TCA) was considered. Subsequent improvement in the lesions was observed following the treatment (Figure 4). The patient was considered for HPV and VZV vaccination for further protection.
Figure 1.
At the patient’s initial visit for treatment, skin-colored verrucous papules were observed on both hands (a) and feet (b).
Figure 2.
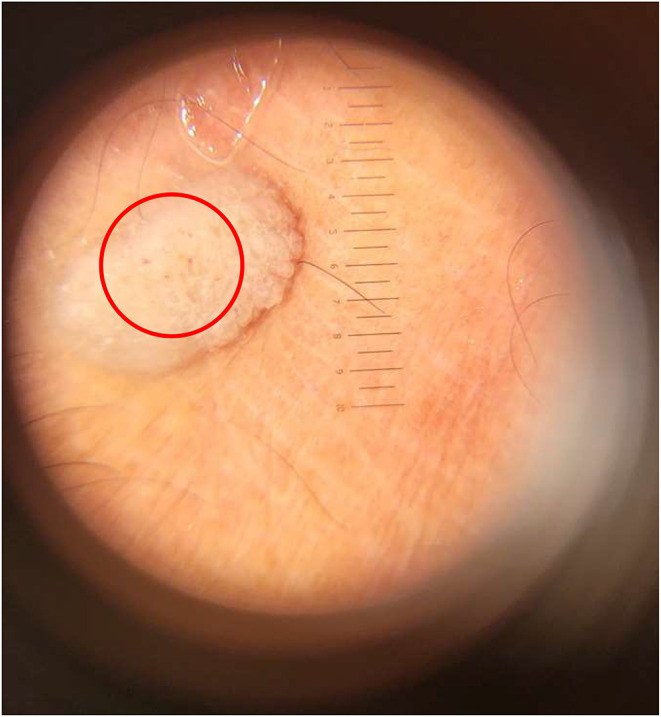
Dermoscopic examination of the verrucous papules revealed dotted vessels.
Figure 3.
Histopathological examination of the verrucous lesions on the feet showed acanthosis (red arrow), hyperkeratosis (green arrow), papillomatosis (black arrow), and the presence of koilocytes (yellow arrow), with no evidence of malignancy.
Figure 4.
By the second week of TCA treatment, partial resolution of the warts was observed on both hands (a) and feet (b). Limitation: a more recent follow-up image was not available.
Discussion
Verruca vulgaris, or common warts, are benign epithelial proliferations caused by the human papillomavirus (HPV).1,24 The incidence peaks between the ages of 12 and 16,24,25 with a higher prevalence in males, particularly those engaged in outdoor physical activities.26,27 HPV infects both mucosal and cutaneous surfaces, with over 150 identified types.5,6 The types that infect the skin are epidermotrophic, targeting keratinized skin surfaces. Meanwhile, mucosal types can infect various mucosal tissues, including the anogenital epithelium.28 HPV types are further categorized into high-risk (HR-HPV) and low-risk (LR-HPV) groups. HR-HPV 16 and 18, are connected to malignancies.20 Different efforts are needed to improve the classification of patients with HPV using genotyping PCR, with a sensitivity of 73.6% and a specificity of 98%.10,29 This method provides valuable information for prognostication and enables patient surveillance.29 In this case report, the patient tested positive for HPV types 4 and 6 (low-risk) as well as type 16 (high-risk).
HPV enters the skin through minor trauma and infects the basal epithelial layer.30 The transmission of genital-type HPV to non-genital skin sites occurs directly through sexual practice including the axilla (maschalagnia or armpit fetishism),29 or direct contact with hands.17 This can be achieved through contaminated objects such as razors or towels,29 or vertical transmission during vaginal delivery with a delayed onset.17 Additionally, individuals with a history of atopy or friction-induced microtrauma to the skin may be more prone to infection.29 As the infection progresses, the infected cells differentiate into keratinocytes and migrate to the epithelial surface. In immunocompetent individuals, the strong immune response typically controls the infection, preventing wart formation.11,20,31 However, in immunocompromised individuals, such as those with HIV, warts are formed due to an inadequate immune response.1,3 Patients with HIV infection show a higher prevalence of HPV infection and tend to experience persistent HPV infections.20 Specific HPV types have a predilection for infecting particular anatomical sites, a tendency influenced by immunological status.18 For instance, HPV types 6 and 11 are found in the anogenital region. Therefore, the appearance of these HPV types in atypical locations may report a failure of the immune defense system.19,32 Additionally, HIV patients are infected with uncommon HPV types and may present with multiple types within a single lesion due to the compromised immune system inability to control expression and replication.9,20 Porro et al9 reported that the duration of wart symptoms in individuals with HIV ranges from 6 months to 10 years. These individuals often experience infections with multiple uncommon HPV types. Besides HIV infection, other factors have a potential role in the atypical presentation of mucosatropic HPV such as type 6 in non-genital skin sites33 such as T-cell inherited cellular immunodeficiency.19 The previous cases occurred at a young age acting as a potential risk factor in the population17,34 Meanwhile, other cases suggested the possibility of immunological impairments but were unable to detect.17,18 The mechanism used by HIV infection to increase the prevalence and severity of HPV is not fully understood. However, the process includes interactions between HIV and HPV proteins.8 Immunological dysfunction in HIV patients significantly impacts the progression of HPV infections. Effective immunity against HPV relies on robust, localized cell-mediated immunity, which is compromised in individuals with HIV. Moreover, HIV infection alters the cytokine expression in epithelial cells, promoting the reactivation of latent viruses in keratinocytes and increasing the progression of existing HPV infections.8 The virus may also induce the expression of pro-inflammatory cytokines such as interleukin (IL)-6, IL-1, and tumor necrosis factor (TNF)-alpha, which can enhance HPV transcription and increase the viral load.11 Due to the advancement in HIV/AIDS, there is a decline in the cytotoxic T-lymphocyte response to HPV oncoproteins E6 and E7, impairing the ability to eliminate HPV and allowing for unchecked epithelial cell proliferation.8 The impairment of cellular immune response to HPV by HIV infection diminished the ability to clear HPV infections, particularly when CD4+ T cell counts fall below 120/uL.9,35 In terms of humoral immunity, seroconversion following HPV vaccination is lower in HIV-positive patients compared to immunocompetent individuals, particularly among those with a CD4 count below 200 cells/mm³.36 In contrast, prevalent HPV infection may increase the risk of HIV acquisition through multiple mechanisms. These include the down-regulation of E-Cadherin by the HPV E7 protein, which increases the susceptibility of the genital tract to HIV. The infection also disrupts the density and morphology of Langerhans cells, reducing the capacity to block HIV infection. Furthermore, the T-lymphocyte-driven immune response to HPV may elevate the risk of HIV. Non-persistent HPV infections, associated with T-lymphocyte influx, have been connected to a higher likelihood of HIV acquisition.37 In this case, the HIV Stage 4 status of the patient, with CD4 level 106/uL increased the severity of the HPV infection, although lack of data on viral load. This report was only on single-patient focus with the possibility of selection bias which became a limitation in generalizing the correlation on further cases. Even though the association between HPV type 6 and skin lesions is evident, establishing a causal relationship with certainty is impossible. The HIV status increases the likelihood of persistent infection, which may be more resistant to treatment due to association with a higher recurrence rate and an elevated risk of progressing to malignancy.
Clinical manifestations of verruca vulgaris often begin asymptomatically, developing into well-defined, thickened hyperkeratotic lesions.1,6 Meanwhile, clinical manifestations of mucosatropic HPV infection including non-genital skin sites are rarely reported.17 Cavalcanti et al19 diagnosed the presence of HPV types 6 and 16 in a 39-year-old immunodeficient male with warts on the hands and feet, without any anogenital lesions. Another case was reported by Blauvelt et al17 which included extragenital warts on the arms and legs caused by HPV type 6 in a 9-year-old girl persisting for three years. Examination showed hyperpigmented verrucous papules across the arms and legs, with no lesions on the oral or genital mucosa. Hsu et al18 described a case of HPV type 6 in a 30-year-old male presenting with warts in the axillary region. The patient was suspected to have immunodeficiency, facilitating the progression of the HPV infection.29 The diagnosis of verruca vulgaris is typically clinical, but histopathological examination is recommended for resistant lesions, particularly in immunocompromised patients.38 Histopathological findings typically include acanthosis, epidermal hyperplasia, papillomatosis, orthokeratosis, hypergranulosis, tortuous capillaries in dermal papillae, and parakeratosis with red blood cells trapped at the tips of digitations.1,38 Infected cells in the granular layer, known as koilocytes, are characterized by coarse keratohyalin granules and perinuclear vacuolization.1,6 Dermoscopic examination can also aid in the diagnosis, showing features such as papilloma growth, finger-like projections, hairpin-like vessels, dotted and linear vessels, and frogspawn-like hemorrhagic spots surrounded by a white halo.39 In this context, the patient was diagnosed with verruca vulgaris based on the clinical history of rough, skin-colored nodules on the hands and feet. The physical examination showed hyperpigmented papules and verrucous plaques, histopathological findings of acanthosis, epidermal hyperplasia, papillomatosis, orthokeratosis, and koilocytes without evidence of malignancy, as well as dermoscopic findings of dotted vessels.
The treatment aims to alleviate physical and psychological discomfort, prevent the spread of infection, and minimize side effects.40 The numerous therapeutic modalities available are not ideal due to the inability to achieve complete eradication of HPV infection,41 leading to insufficient prevention of recurrence.42,43 The choice of treatment depends on factors such as the location, size, and number of warts, patient preference, cost, convenience, side effects, available instruments, and the experience of the physician.41,42 Treatment options fall into four categories of cytodestructive methods, antiviral therapy (cidofovir), chemotherapeutic agents (bleomycin and 5-fluorouracil), and immunotherapy (imiquimod, zinc sulfate, cimetidine). Cryotherapy induces necrosis of HPV-infected keratinocytes or stimulates a local inflammatory response to trigger immune reaction.38 Success rates for cryotherapy range from 50% to 70%, with treatment typically administered every two to three weeks for three months.44 However, side effects such as pain, blister formation, scarring, and changes in pigmentation can occur.27,41 After the fourth cryotherapy session, the patient reported intolerable pain and blistering, leading to a switch to 80% TCA. The chemical agent destroys tissue through cellular protein hydrolysis, with success rates of 70% to 81%.43,45 TCA applied up to six times over six to eight weeks can cause side effects such as pain, infection, ulceration, and scarring.46 Moreover, the application is important for HPV vaccination, especially in fragile individuals affected by HIV. HPV vaccines have proved to have outstanding safety, including in HIV patients with lower efficacy than in HIV-negative individuals.47 There is also growing evidence of the possibility of therapeutic effects of HPV vaccination on AGWs and cutaneous warts.48 The immunization of VZV in HIV infected patients is safe with a preventative measure for varicella and herpes zoster.49 In this context, the patient experienced partial wart removal by the second week with minimal pain and continue the therapy, as reported in Figure 4. The patient is still considering HPV and varicella zoster virus vaccination for further protection.
Conclusion
In conclusion, HPV genotyping was crucial in immunocompromised patients, particularly those with HIV, who were susceptible to persistent infections with atypical types. The identification of low-risk and high-risk HPV strains in non-genital areas suggested a compromised immune response, increasing the risk of progression to malignancy. Therefore, thorough diagnostic evaluations, including PCR testing, were essential for effectively managing and monitoring HPV in immunocompromised individuals with suggestions for HPV prevention through available vaccination.
Acknowledgments
The authors are grateful to the Departments of Dermatology and Venereology personnel at the Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin General Hospital.
Ethical Statement
Written informed consent was provided by the patient to have the case details and any accompanying images published. Institutional approval was obtained to publish the case details from Dr. Hasan Sadikin Hospital Ethical Committee with ethical approval number DP.04.03/D.XIV.6.5/412/2024.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Al Aboud AM, Nigam PK. Wart. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. PMID: 28613701. [Google Scholar]
- 2.Soenjoyo KR, Chua BWB, Wee LWY, Koh MJA, Ang SB. Treatment of cutaneous viral warts in children: a review. Dermatol Ther. 2020;33(6):e14034. doi: 10.1111/dth.14034 [DOI] [PubMed] [Google Scholar]
- 3.Sterling JC. Virus Infections. Dalam: Rook’s Textbook of Dermatology. John Wiley & Sons, Ltd; 2010:hlm.1–8. [Google Scholar]
- 4.Salah E. Impact of multiple extragenital warts on quality of life in immune-competent Egyptian adults: a comparative cross-sectional study. Clin Cosmet Invest Dermatol. 2018;11:289–295. doi: 10.2147/CCID.S165908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez BY, Shvetsov YB, Goodman MT, et al. Genital and extra-genital warts increase the risk of asymptomatic genital human papillomavirus infection in men. Sex Transm Infect. 2011;87(5):391–395. doi: 10.1136/sti.2010.048876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterling JC. Human papillomavirus infections. In: Kang S, Amagai M, Bruckner AL, et al., editors. Penyunting. Fitzpatrick’s Dermatology, 9e. New York, NY: McGraw-Hill Education; 2019. [Google Scholar]
- 7.Redzic N, Pereira AR, Menon S, et al. Characterization of type-specific HPV prevalence in a population of persistent cutaneous warts in Flanders, Belgium. Sci Rep. 2023;13(1):17492. doi: 10.1038/s41598-023-44154-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gormley RH, Kovarik CL. Dermatologic manifestations of HPV in HIV-infected individuals. Curr HIV/AIDS Rep. 2009;6(3):130–138. doi: 10.1007/s11904-009-0018-8 [DOI] [PubMed] [Google Scholar]
- 9.Porro AM, Alchorne MM, Mota GR, Michalany N, Pignatari AC, Souza IE. Detection and typing of human papillomavirus in cutaneous warts of patients infected with human immunodeficiency virus type 1. Br J Dermatol. 2003;149(6):1192–1199. doi: 10.1111/j.1365-2133.2003.05650.x [DOI] [PubMed] [Google Scholar]
- 10.Pesic A, Krings A, Schreckenberger C, Hempel M, Preyer R, Kaufmann AM. Analytical evaluation of the human papillomavirus HPV DNA array el-based genotyping assay. Intervirology. 2019;62(3):124–133. doi: 10.1159/000502207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verssimo J, de Medeiros Fernandes TA. Human papillomavirus: biology and pathogenesis. InTech. 2012;12(1):1–27. [Google Scholar]
- 12.Yu YB, Wang YH, Yang XC, et al. The relationship between human papillomavirus and penile cancer over the past decade: a systematic review and meta-analysis. Asian J Androl. 2019;21(4):375–380. PMID: 31134917; PMCID: PMC6628743. doi: 10.4103/aja.aja_39_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidd LC, Chaing S, Chipollini J, Giuliano AR, Spiess PE, Sharma P. Relationship between human papillomavirus and penile cancer-implications for prevention and treatment. Transl Androl Urol. 2017;6(5):791–802. PMID: 29184775; PMCID: PMC5673821. doi: 10.21037/tau.2017.06.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute. HPV and Cancers. 2024. Available from: https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer. Accessed January 9, 2025.
- 15.World Health Organization. Human papillomavirus and Cancer. Available from: https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer. Accessed January 9, 2025.
- 16.Achdiat PA, Suwarsa O, Hidayat YM, et al. Efficacy and safety profile of tuberculin protein purified derivative injection as immunotherapy for the treatment of cutaneous and anogenital warts: a review article. Immunotargets Ther. 2024;13:123–150. doi: 10.2147/ITT.S446938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blauvelt A, Duarte AM, Pruksachatkunakorn C, Leonardi CL, Schachner LA. Human papillomavirus type 6 infection involving cutaneous nongenital sites. J Am Acad Dermatol. 1992;27(5):876–879. doi: 10.1016/0190-9622(92)70271-G [DOI] [PubMed] [Google Scholar]
- 18.Hsu T, Nahmias ZP, Rosman IS, Sheinbein D. Extragenital condyloma acuminatum in the left axillary vault. JAAD Case Rep. 2018;4(9):947–949. doi: 10.1016/j.jdcr.2018.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavalcanti SM, Deus FC, Oliverira LH. Unusual HPV types in cutaneous warts in association with immunological deficiency. Mem Inst Oswaldo Cruz Rio de Janeiro. 1998;93(4):433–434. doi: 10.1590/S0074-02761998000400002 [DOI] [PubMed] [Google Scholar]
- 20.Marchetti G, Comi L, Bini T, et al. HPV infection in a cohort of hiv-positive men and women: prevalence of oncogenic genotypes and predictors of mucosal damage at genital and oral sites. J Sex Transm Dis. 2013;5(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akbari E, Milani A, Seyedinkhorasani M, Bolhassani A. HPV co-infections with other pathogens in cancer development: a comprehensive review. J Med Virol. 2023;95(11):e29236. PMID: 37997472. doi: 10.1002/jmv.29236 [DOI] [PubMed] [Google Scholar]
- 22.Silva LLD, Teles AM, Santos JMO, et al. Malignancy associated with low-risk HPV6 and HPV11: a systematic review and implications for cancer prevention. Cancers. 2023;15(16):4068. PMID: 37627099; PMCID: PMC10452364. doi: 10.3390/cancers15164068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suominen H, Syrjänen K, Waterboer T, Grénman S, Syrjänen S, Louvanto K. Serum IgG antibodies to HPV6 L1, E2, E4, E6, and E7 proteins among children prospectively followed-up for three years. J Infect Dis. 2024:jiae293. PMID: 38820118. doi: 10.1093/infdis/jiae293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur S, Kumar S, Brar BK, Brar SK. Study of socio-demographic details of verruca vulgaris patients—an institutional based study. J Adv Dent Res. 2017;5(3):87–90. [Google Scholar]
- 25.Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or candida antigens. Pediatr Dermatol. 2003;20(3):268–271. doi: 10.1046/j.1525-1470.2003.20318.x [DOI] [PubMed] [Google Scholar]
- 26.Asghar M. Epidemiological and clinical patterns of viral warts presenting to dermatology OPD of Hayatabad Medical Complex, Peshawar. J Pak Assoc Dermatol. 2022;32(1):78–84. [Google Scholar]
- 27.Fadila A, Zulkarnain I, Yulianto Listiawan M, Nurul hidayati A, Mira Indramaya D, Utomo B. A retrospective study of verruca. Berkala Ilmu Kesehatan Kulit Dan Kelamin. 2022;34(2):77–80. doi: 10.20473/bikk.V34.2.2022.77-80 [DOI] [Google Scholar]
- 28.Egawa N, Egawa K, Griffin H, Doorbar J. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses. 2015;7(7):2863–2890. doi: 10.3390/v7072802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Achdiat PA, Septharina R, Rowawi R, et al. A review and case study of genital and extragenital human papillomavirus type 6 and 11 infections in men who have sex with men accompanied by human immunodeficiency virus infection. HIV/AIDS-Res Palliative Care. 2024;16:175–182. doi: 10.2147/HIV.S451989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magalhães GM, Vieira ÉC, Garcia LC, et al. update on human papilloma virus - part i: epidemiology, pathogenesis, and clinical spectrum. An Bras Dermatol. 2021;96(1):1–16. doi: 10.1016/j.abd.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reusser NM, Downing C, Guidry J, Tyring SK. HPV carcinomas in immunocompromised patients. J Clin Med. 2015;4(2):260–281. doi: 10.3390/jcm4020260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruggink SC, Koning MN, Gussekloo J, et al. Cutaneous wart-associated HPV types: prevalence and relation with patient characteristics. J Clin Virol. 2012;55(3):250–255. doi: 10.1016/j.jcv.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 33.Shamanin V, Glover M, Rausch C, et al. Specific types of human papillomavirus found in benign proliferations and carcinomas of the skin in immunosuppressed patients. Cancer Res. 1994;54:4610–4613. [PubMed] [Google Scholar]
- 34.Payne D, Ramon S, Stephen T. Cutaneous verruca with genital human papillomavirus in a 2-year-old girl. Ped Dermatol. 1997;19(3):258–260. [DOI] [PubMed] [Google Scholar]
- 35.Dolev JC, Maurer T, Springer G, et al. Incidence and risk factors for verrucae in women. AIDS. 2008;22(10):1213–1219. doi: 10.1097/QAD.0b013e3283021aa3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Losada C, Samaha H, Scherer EM, et al. Efficacy and durability of immune response after receipt of HPV vaccines in people living with HIV. Vaccines. 2023;11(6):1067. doi: 10.3390/vaccines11061067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houlihan CF, Larke NL, Watson-Jones D, et al. Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. AIDS. 2012;26(17):2211–2222. PMID: 22874522; PMCID: PMC3831022. doi: 10.1097/QAD.0b013e328358d908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipke MM. An armamanterium of wart treatments. Clin Med Res. 2006;4(4):273–293. doi: 10.3121/cmr.4.4.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al Rudaisat M, Cheng H. dermoscopy features of cutaneous warts. Int J Gen Med. 2021;14:9903–9912. doi: 10.2147/IJGM.S335276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalimunthe DA, Tanjung C. The relation between healing time and patient’s characteristic in the treatment of common warts with the application of 80% phenol solution. Berkala Ilmu Kesehatan Kulit Dan Kelamin. 2016;28(1):23–26. [Google Scholar]
- 41.Zhu P, Qi RQ, Yang Y, et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J Evid Based Med. 2022;15(3):284–301. doi: 10.1111/jebm.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aldahan AS, Mlacker S, Shah VV, et al. Efficacy of intralesional immunotherapy for the treatment of warts: a review of the literature. Dermatol Ther. 2016;29(3):197–207. doi: 10.1111/dth.12352 [DOI] [PubMed] [Google Scholar]
- 43.Pezeshkpoor F, Banihashemi M, Yazdanpanah MJ, Yousefzadeh H, Sharghi M, Hoseinzadeh H. Comparative study of topical 80% trichloroacetic acid with 35% trichloroacetic acid in the treatment of the common wart. J Drugs Dermatol. 2012;11(11):e66–9. [PubMed] [Google Scholar]
- 44.Liu J, Li H, Yang F, et al. Epidemiology and clinical profile of cutaneous warts in Chinese college students: a cross-sectional and follow-up study. Sci Rep. 2018;8(1):524–562. doi: 10.1038/s41598-017-18861-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pramita N, Winaya K, Darmaputra I. Recurrent verruca vulgaris treated with combination of 80% trichloroacetate and electrosurgery: a case report. Berkala Ilmu Kesehatan Kulit Dan Kelamin. 2022;34(1):73–76. doi: 10.20473/bikk.V34.1.2022.73-76 [DOI] [Google Scholar]
- 46.Dhamayanti ME, Rahayu T, Wirawan EP, Diana R, Yuliarto D, Sari EY. Terapi kondiloma akuminata dengan kombinasi trichloroacetic acid (TCA) 80% + krioterapi. Cermin Dunia Kedokteran. 2019;46(6):443–447. [Google Scholar]
- 47.Lacey CJ. HPV vaccination in HIV infection. Papillomavirus Res. 2019;8:100174. PMID: 31252073; PMCID: PMC6603434. doi: 10.1016/j.pvr.2019.100174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herzum A, Ciccarese G, Occella C, et al. Treatment of pediatric anogenital warts in the era of HPV-vaccine: a literature review. J Clin Med. 2023;12(13):4230. PMID: 37445264;PMCID: PMC10342328. doi: 10.3390/jcm12134230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gershon AA. Prevention and treatment of VZV infections in patients with HIV. Herpes. 2001;8(2):32–36. PMID: 11867015. [PubMed] [Google Scholar]