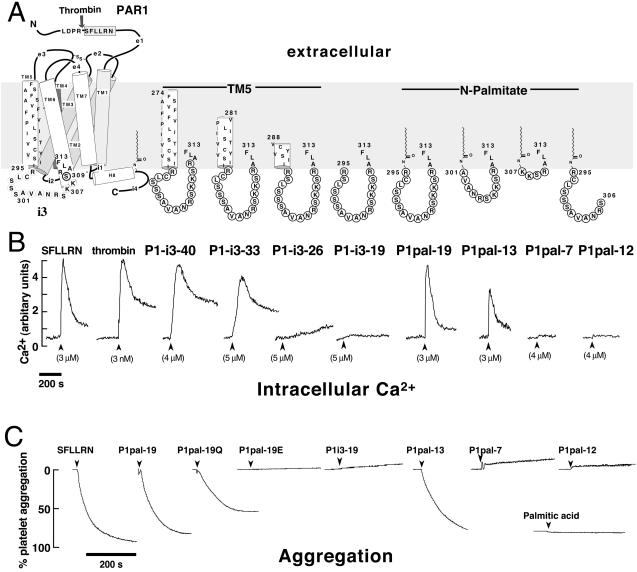
Figure 1.
Membrane-tethered PAR1 i3 loop peptides activate regulated Ca2+ signaling and aggregation in platelets. (A) The topological arrangement of the membrane-spanning segments (TM1–7), extracellular loops (e1-e4), and intracellular loops (i1-i4) of PAR1 is based on the x-ray structure of rhodopsin (1) and is illustrated on the left. Thrombin cleaves the extracellular domain (e1) at the R41—S42 bond creating a new N terminus, S42FLLRN, which functions as a tethered PAR1 agonist. The composition of the peptides used in this study is shown on the right, and their corresponding effects on platelet Ca2+ are shown immediately below. (B) Platelets from healthy volunteer donors were isolated by gel filtration chromatography, and Ca2+ measurements were performed as described (11). Intracellular Ca2+ concentration was monitored as the ratio of fluorescence excitation intensity at 340/380 nm. (C) PAR1 i3 loop peptides cause full platelet aggregation. Individual aggregation traces of platelets stimulated with 10 μM of indicated peptides or 200 μM palmitic acid are shown. Platelet aggregation was monitored as percent of light transmittance of stirred platelets at 37°C, as described (19).