Figure 3.
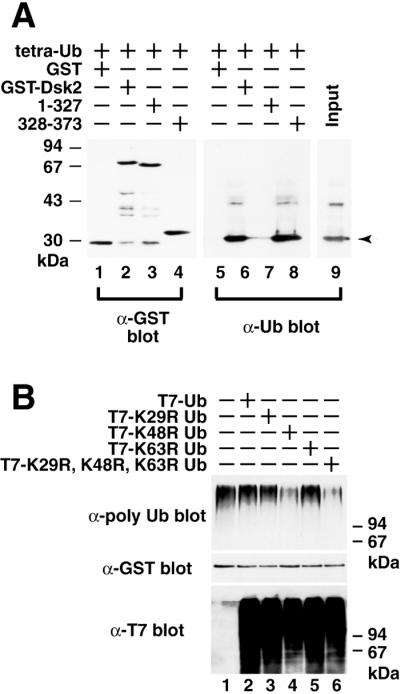
The UBA domain of Dsk2p binds directly to K48-linked polyubiquitin chains. (A) Direct binding of Dsk2p to tetra-Ub. K48-linked tetra-Ub was incubated in vitro with full-length GST-Dsk2p (residues 1–373), C-terminal truncated GST-Dsk2p (residues 1–327), and C-terminal UBA (residues 328–373). GST pull-downs were immunoblotted with anti-GST (lanes 1–4) and anti-Ub (lanes 5–8) antibodies. The arrow in lane 9 indicates the position of tetra-Ub. (B) Effects of Ub lysine mutants on binding between Dsk2p and polyubiquitin. T7-tagged lysine mutants of Ub (K29R, K48R, K63R, and K29R-K48R-K63R) were expressed in yeast (Bottom), and the cell extracts were incubated with recombinant GST-Dsk2p. GST-bound materials were immunoblotted with anti-polyubiquitin (Top) and anti-GST (Middle) antibodies for GST-Dsk2p.
