Abstract
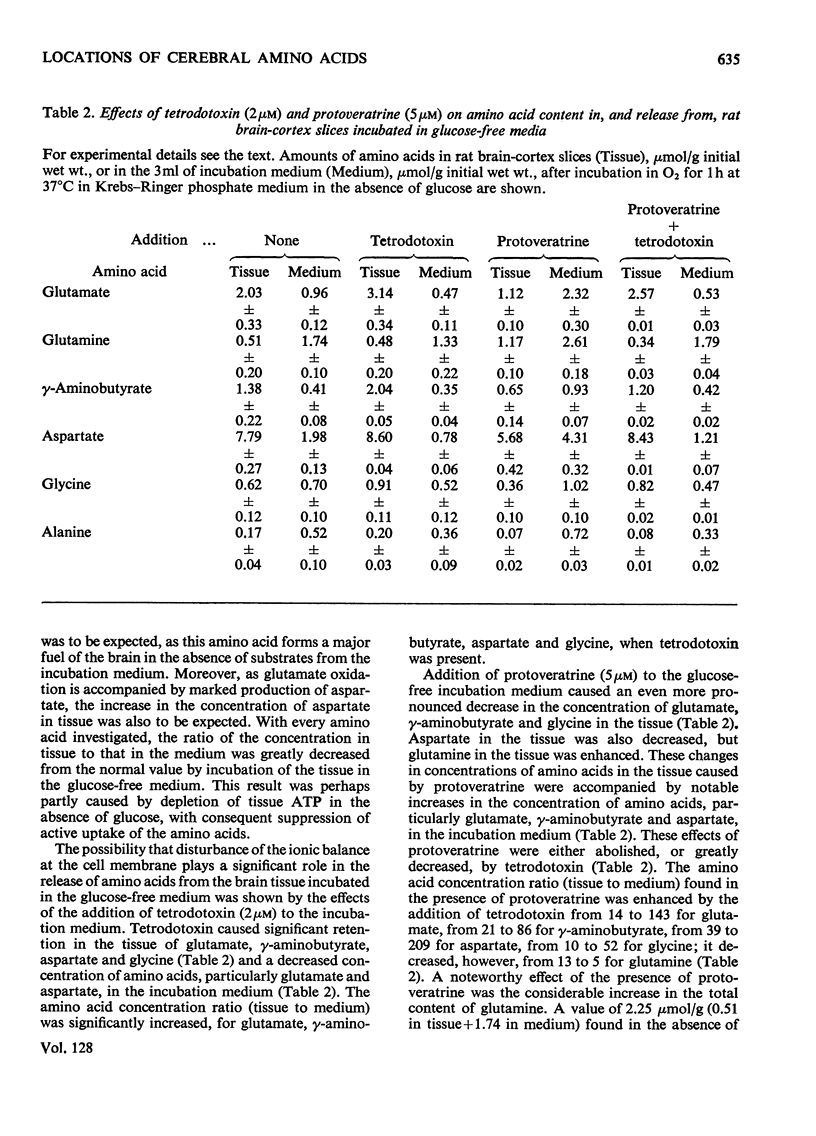
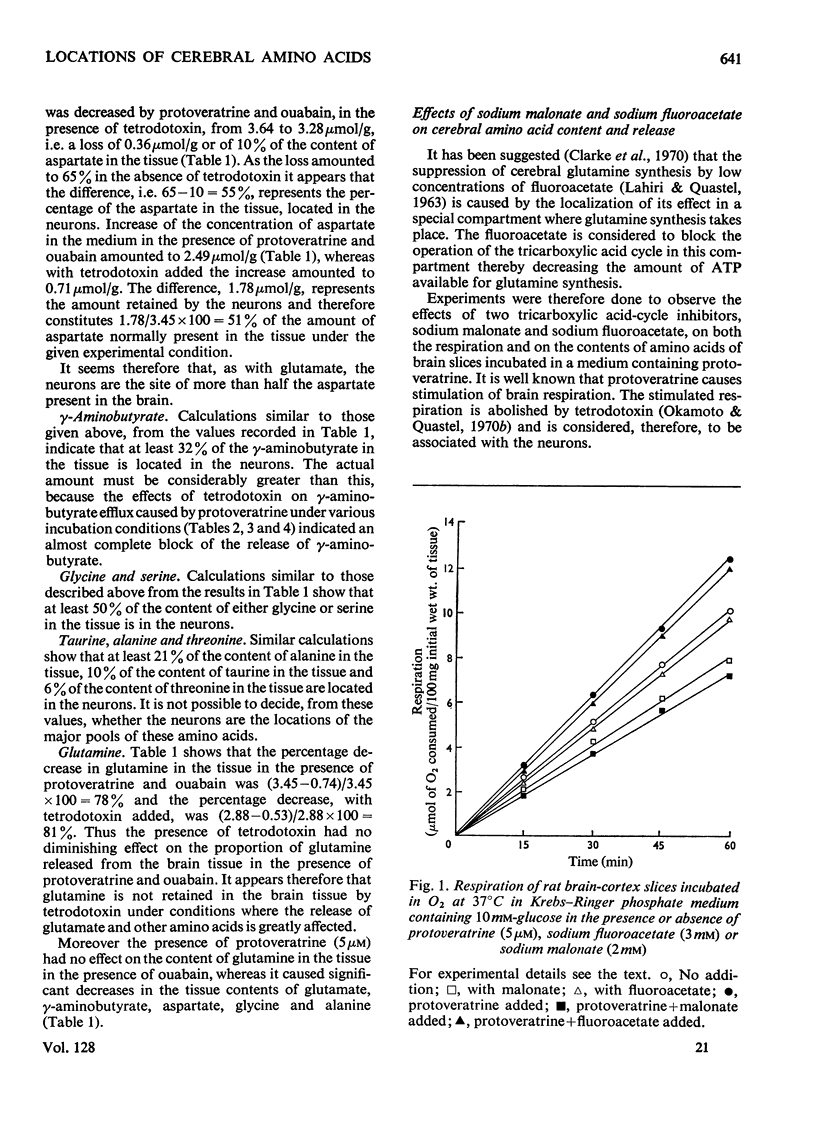
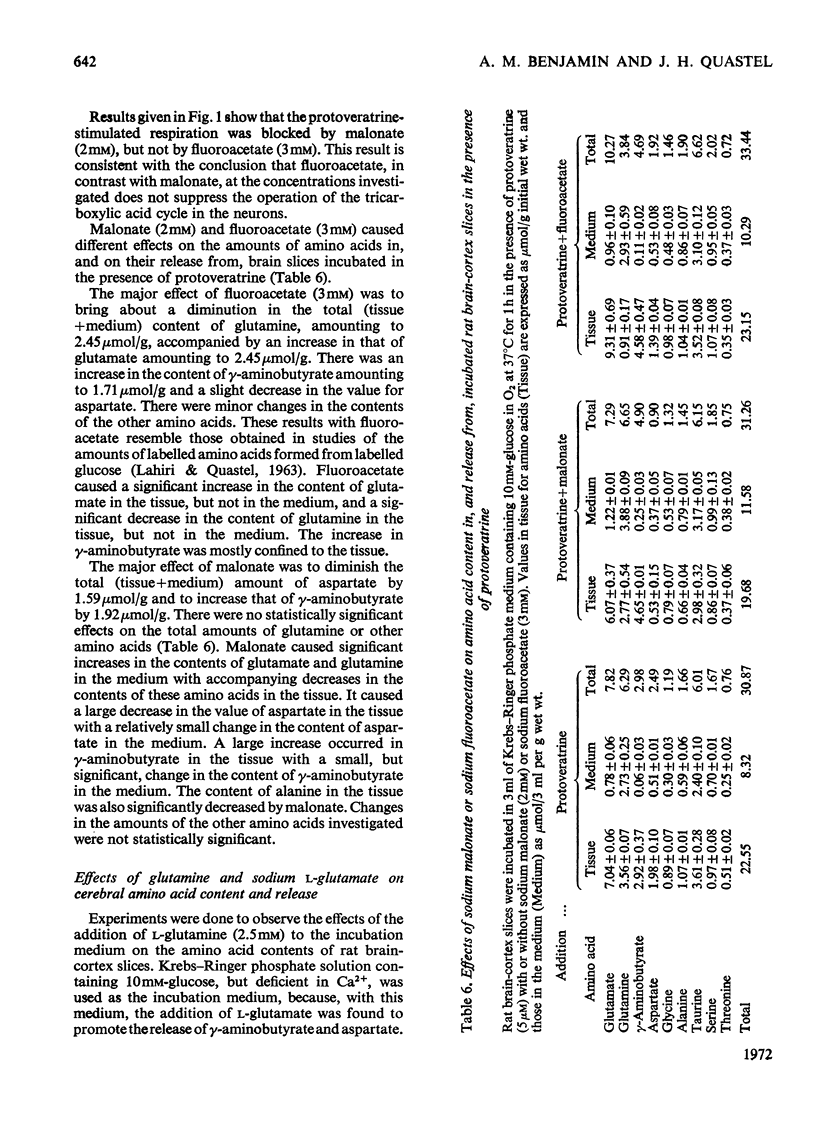
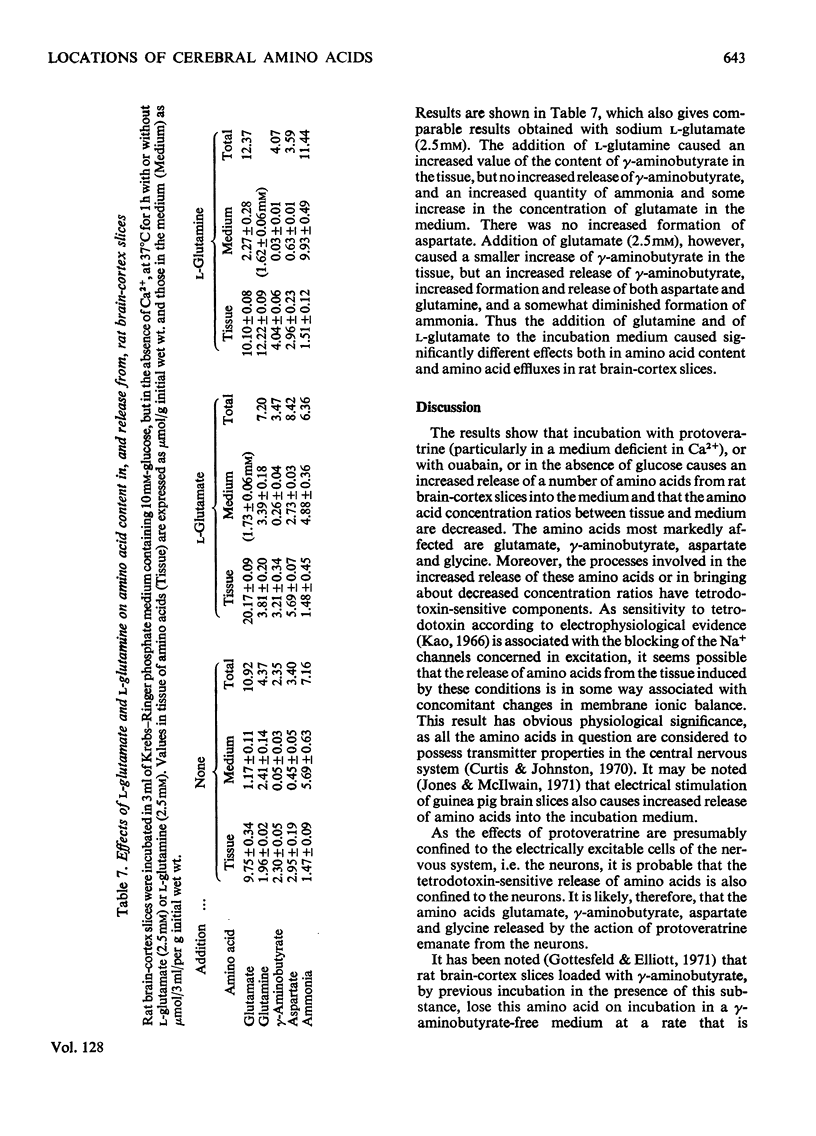
1. Amino acids, particularly glutamate, γ-aminobutyrate, aspartate and glycine, were released from rat brain slices on incubation with protoveratrine (especially in a Ca2+-deficient medium) or with ouabain or in the absence of glucose. Release was partially or wholly suppressed by tetrodotoxin. 2. Tetrodotoxin did not affect the release of glutamine under various incubation conditions, nor did protoveratrine accelerate it. 3. Protoveratrine caused an increased rate of formation of glutamine in incubated brain slices. 4. Increased K+ in the incubation medium caused release of γ-aminobutyrate, the process being partly suppressed by tetrodotoxin. 5. Incubation of brain slices in a glucose-free medium led to increased production of aspartate and to diminished tissue contents of glutamates, glutamine and glycine. 6. Use of tetrodotoxin to suppress the release of amino acids from neurons in slices caused by the joint action of protoveratrine and ouabain (the latter being added to diminish reuptake of amino acids), it was shown that the major pools of glutamate, aspartate, glycine, serine and probably γ-aminobutyrate are in the neurons. 7. The major pool of glutamine lies not in the neurons but in the glia. 8. The tricarboxylic cycle inhibitors, fluoroacetate and malonate, exerted different effects on amino acid contents in, and on amino acid release from, brain slices incubated in the presence of protoveratrine. Fluoroacetate (3mm) diminished the content of glutamine, increased that of glutamate and γ-aminobutyrate and did not affect respiration. Malonate (2mm) diminished aspartate and γ-aminobutyrate content, suppressed respiration and did not affect glutamine content. It is suggested that malonate acts mainly on the neurons, and that fluoroacetate acts mainly on the glia, at the concentrations quoted. 9. Glutamine was more effective than glutamate as a precursor of γ-aminobutyrate. 10. It is suggested that glutamate released from neurons is partly taken up by glia and converted there into glutamine. This is returned to the neurons where it is hydrolysed and converted into glutamate and γ-aminobutyrate.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABADOM P. N., SCHOLEFIELD P. G. Amino acid transport in brain cortex slices. III. The utilization of energy for transport. Can J Biochem Physiol. 1962 Nov;40:1603–1618. [PubMed] [Google Scholar]
- Balázs R., Dahl D., Harwood J. R. Subcellular distribution of enzymes of glutamate metabolism in rat brain. J Neurochem. 1966 Oct;13(10):897–905. doi: 10.1111/j.1471-4159.1966.tb10285.x. [DOI] [PubMed] [Google Scholar]
- Balázs R., Machiyama Y., Hammond B. J., Julian T., Richter D. The operation of the gamma-aminobutyrate bypath of the tricarboxylic acid cycle in brain tissue in vitro. Biochem J. 1970 Feb;116(3):445–461. doi: 10.1042/bj1160445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl S., Frigyesi T. L. Comparison of cerebral regional metabolism of [14C]leucine following third ventricle and intravenous administration in the cat. J Neurochem. 1969 Mar;16(3):405–415. doi: 10.1111/j.1471-4159.1969.tb10381.x. [DOI] [PubMed] [Google Scholar]
- Berl S., Nicklas W. J., Clarke D. D. Compartmentation of glutamic acid metabolism in brain slices. J Neurochem. 1968 Feb;15(2):131–140. doi: 10.1111/j.1471-4159.1968.tb06184.x. [DOI] [PubMed] [Google Scholar]
- CARVER M. J. INFLUENCE OF PHENYLALANINE ADMINISTRATION ON THE FREE AMINO ACIDS OF BRAIN AND LIVER IN THE RAT. J Neurochem. 1965 Jan;12:45–50. doi: 10.1111/j.1471-4159.1965.tb10250.x. [DOI] [PubMed] [Google Scholar]
- CRAVIOTO R. O., MASSIEU G., IZQUIERDO J. J. Free amino-acids in rat brain during insulin shock. Proc Soc Exp Biol Med. 1951 Dec;78(3):856–858. doi: 10.3181/00379727-78-19241. [DOI] [PubMed] [Google Scholar]
- Chan S. L., Quastel J. H. Tetrodotoxin: effects on brain metabolism in vitro. Science. 1967 Jun 30;156(3783):1752–1753. doi: 10.1126/science.156.3783.1752. [DOI] [PubMed] [Google Scholar]
- Cherayil A., Kandera J., Lajtha A. Cerebral amino acid transport in vitro. IV. The effect of inhibitors on exit from brain slices. J Neurochem. 1967 Jan;14(1):105–115. doi: 10.1111/j.1471-4159.1967.tb09499.x. [DOI] [PubMed] [Google Scholar]
- Clarke D. D., Nicklas W. J., Berl S. Tricarboxylic acid-cycle metabolism in brain. Effect of fluoroacetate and fluorocitrate on the labelling of glutamate, aspartate, glutamine and gamma-aminobutyrate. Biochem J. 1970 Nov;120(2):345–351. doi: 10.1042/bj1200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks J. A., Balázs R., Johnson A. L., Eayrs J. T. Effect of thyroid hormone on the biochemical maturation of rat brain: conversion of glucose-carbon into amino acids. J Neurochem. 1970 Aug;17(8):1275–1285. doi: 10.1111/j.1471-4159.1970.tb03376.x. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. Cerebral amino acids in fluoroacetate-poisoned, anaesthetised and hypoglycaemic rats. Biochim Biophys Acta. 1953 Aug;11(4):548–552. doi: 10.1016/0006-3002(53)90094-8. [DOI] [PubMed] [Google Scholar]
- Flock E. V., Tyce G. M., Owen C. A., Jr Utilization of [U-14C]glucose in brain after total hepatectomy in the rat. J Neurochem. 1966 Dec;13(12):1389–1406. doi: 10.1111/j.1471-4159.1966.tb04301.x. [DOI] [PubMed] [Google Scholar]
- GIBSON I. M., MCILWAIN H. CONTINUOUS RECORDINGS OF CHANGES IN MEMBRANE POTENTIAL IN MAMMALIAN CEREBRAL TISSUES IN VITRO; RECOVERY AFTER DEPOLARIZATION BY ADDED SUBSTANCES. J Physiol. 1965 Jan;176:261–283. doi: 10.1113/jphysiol.1965.sp007549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONDA O., QUASTEL J. H. Effects of ouabain on cerebral metabolism and transport mechanisms in vitro. Biochem J. 1962 Aug;84:394–406. doi: 10.1042/bj0840394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONDA O., QUASTEL J. H. Succinate/amino-acid interrelations in rat brain cortex in vitro. Nature. 1962 Jan 13;193:138–140. doi: 10.1038/193138a0. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. A simulation study of the metabolism and compartmentation in brain of glutamate, aspartate, the Krebs cycle, and related metabolites. J Biol Chem. 1966 Sep 10;241(17):3918–3929. [PubMed] [Google Scholar]
- Gottesfeld Z., Elliott K. A. Factors that affect the binding and uptake of gaba by brain tissue. J Neurochem. 1971 May;18(5):683–690. doi: 10.1111/j.1471-4159.1971.tb11998.x. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. A., McIlwain H. Amino acid production and translocation in incubated and superfused tissues from the brain. J Neurobiol. 1971;2(4):311–326. doi: 10.1002/neu.480020404. [DOI] [PubMed] [Google Scholar]
- KINI M. M., QUASTEL J. H. Effects of veratrine and cocaine on cerebral carbohydrate-amino acid interrelations. Science. 1960 Feb 12;131(3398):412–414. doi: 10.1126/science.131.3398.412. [DOI] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Kuffler S. W. Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci. 1967 Jun 6;168(1010):1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- Kuhar M. J., Snyder S. H. The subcellular distribution of free H3-glutamic acid in rat cerebral cortical slices. J Pharmacol Exp Ther. 1970 Jan;171(1):141–152. [PubMed] [Google Scholar]
- LAHIRI S., QUASTEL J. H. FLUOROACETATE AND THE METABOLISM OF AMMONIA IN BRAIN. Biochem J. 1963 Oct;89:157–163. doi: 10.1042/bj0890157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiyama Y., Balázs R., Hammond B. J., Julian T., Richter D. The metabolism of gamma-aminobutyrate and glucose in potassium ion-stimulated brain tissue in vitro. Biochem J. 1970 Feb;116(3):469–481. doi: 10.1042/bj1160469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiyama Y., Balázs R., Richter D. Effect of K+-stimulation on GABA metabolism in brain slices in vitro. J Neurochem. 1967 May;14(5):591–594. doi: 10.1111/j.1471-4159.1967.tb09560.x. [DOI] [PubMed] [Google Scholar]
- Margolis R. K., Heller A., Moore R. Y. Effects of changes in cellular composition following neuronal degeneration on amino acids in brain. Brain Res. 1968 Oct;11(1):19–31. doi: 10.1016/0006-8993(68)90071-1. [DOI] [PubMed] [Google Scholar]
- McIlwain H. Tetrodotoxin and the cation content, excitability and metabolism of isolated mammalian cerebral tissues. Biochem Pharmacol. 1967 Aug;16(8):1389–1396. doi: 10.1016/0006-2952(67)90114-1. [DOI] [PubMed] [Google Scholar]
- Mukherji B., Turinsky J., Sloviter H. A. Effects of perfusion without glucose on amino acids and glycogen of isolated rat brain. J Neurochem. 1971 Sep;18(9):1783–1785. doi: 10.1111/j.1471-4159.1971.tb03756.x. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Nakajima S., Grundfest H. The action of tetrodotoxin on electrogenic components of squid giant axons. J Gen Physiol. 1965 Jul;48(6):975–996. [PubMed] [Google Scholar]
- Nakazawa S., Quastel J. H. Effects of inorganic salts and of ouabain on some metabolic responses of rat cerebral cortex slices to cationic and electrical stimulations. Can J Biochem. 1968 Apr;46(4):355–362. doi: 10.1139/o68-052. [DOI] [PubMed] [Google Scholar]
- Neal M. J., Iversen L. L. Subcellular distribution of endogenous and (3H) gamma-aminobutyric acid in rat cerebral cortex. J Neurochem. 1969 Aug;16(8):1245–1252. doi: 10.1111/j.1471-4159.1969.tb05972.x. [DOI] [PubMed] [Google Scholar]
- Nicklas W. J., Clarke D. D. Decarboxylation studies of glutamate, glutamine, and aspartate from brain labelled with [1-14C]acetate, L-[U-14C]-aspartate, and L-[U-14C]glutamate. J Neurochem. 1969 Apr;16(4):549–558. doi: 10.1111/j.1471-4159.1969.tb06854.x. [DOI] [PubMed] [Google Scholar]
- O'Neal R. M., Koeppe R. E. Precursors in vivo of glutamate, aspartate and their derivatives of rat brain. J Neurochem. 1966 Sep;13(9):835–847. doi: 10.1111/j.1471-4159.1966.tb05879.x. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Quastel J. H. Tetrodotoxin-sensitive uptake of ions and water byslices of rat brain in vitro. Biochem J. 1970 Nov;120(1):37–47. doi: 10.1042/bj1200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Quastel J. H. Water uptake and energy metabolism in brain slices from the rat. Biochem J. 1970 Nov;120(1):25–36. doi: 10.1042/bj1200025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A. J., Balázs R. Effect of thyroid hormone on metabolic compartmentation in the developing rat brain. Biochem J. 1971 Feb;121(3):469–481. doi: 10.1042/bj1210469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli F., Grynbaum A., Lajtha A. Developmental changes in Na + , K + and ATP and in the levels and transport of amino acids in incubated slices of rat brain. J Neurochem. 1971 Jun;18(6):1135–1148. doi: 10.1111/j.1471-4159.1971.tb12042.x. [DOI] [PubMed] [Google Scholar]
- Quastel J. H. Molecular transport at cell membranes. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):169–196. doi: 10.1098/rspb.1965.0065. [DOI] [PubMed] [Google Scholar]
- ROBERTS S., MORELOS B. S. REGULATION OF CEREBRAL METABOLISM OF AMINO ACIDS. IV. INFLUENCE OF AMINO ACID LEVELS ON LEUCINE UPTAKE, UTILIZATION AND INCORPORATION INTO PROTEIN IN VIVO. J Neurochem. 1965 May;12:373–387. doi: 10.1111/j.1471-4159.1965.tb04238.x. [DOI] [PubMed] [Google Scholar]
- SALGANICOFF L., DEROBERTIS E. SUBCELLULAR DISTRIBUTION OF THE ENZYMES OF THE GLUTAMIC ACID, GLUTAMINE AND GAMMA-AMINOBUTYRIC ACID CYCLES IN RAT BRAIN. J Neurochem. 1965 Apr;12:287–309. doi: 10.1111/j.1471-4159.1965.tb06766.x. [DOI] [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. II. The action potential and excitation. Pharmacol Rev. 1958 Jun;10(2):165–273. [PubMed] [Google Scholar]
- Shankar R., Quastel J. H. Effects of tetrodotoxin and anaesthetics on brain metabolism and transport during anoxia. Biochem J. 1972 Feb;126(4):851–867. doi: 10.1042/bj1260851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V., Neal M. J., Mitchell J. F. The effect of electrical stimulation and high potassium concentrations on the efflux of (3H) gamma-aminobutyric acid from brain slices. J Neurochem. 1969 Aug;16(8):1235–1244. doi: 10.1111/j.1471-4159.1969.tb05971.x. [DOI] [PubMed] [Google Scholar]
- Ulbricht W. The effect of veratridine on excitable membranes of nerve and muscle. Ergeb Physiol. 1969;61:18–71. doi: 10.1007/BFb0111446. [DOI] [PubMed] [Google Scholar]
- Van den Berg C. J., Krzalić L., Mela P., Waelsch H. Compartmentation of glutamate metabolism in brain. Evidence for the existence of two different tricarboxylic acid cycles in brain. Biochem J. 1969 Jun;113(2):281–290. doi: 10.1042/bj1130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAELSCH H., BERL H. W., ROSSI C. A., CLARKE D. D., PURPURA D. P. QUANTITATIVE ASPECTS OF CO2 FIXATION IN MAMMALIAN BRAIN IN VIVO. J Neurochem. 1964 Oct;11:717–728. doi: 10.1111/j.1471-4159.1964.tb06117.x. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A. Action of protoveratrine on the metabolism of cerebral cortex. 1. Unstimulated cerebral-cortex tissue. Biochem J. 1955 Sep;61(1):68–77. doi: 10.1042/bj0610068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A. Action of protoveratrine on the metabolism of cerebral cortex. 2. Electrically stimulated cerebral-cortex tissue. Biochem J. 1955 Sep;61(1):77–80. doi: 10.1042/bj0610077. [DOI] [PMC free article] [PubMed] [Google Scholar]


