Abstract
Introduction
Mucinous appendiceal neoplasms are unique tumors in which >50 % of the tumor volume is composed of extracellular mucin. They may present as an unruptured mucin-filled appendix or, more commonly, with peritoneal metastases after rupture or transmural invasion of the primary tumor. This case report describes a case of presumed ovarian malignancy with final pathologic diagnosis of low grade appendiceal mucinous neoplasm. Due to its rarity, we decided to report it.
Case presentation
A 37-year-old patient presented with a compliant of abdominal swelling. Abdominopelvic ultrasound was done and showed huge right and left complex cystic ovarian masses having thick septa. For this, she underwent total abdominal hysterectomy, omentectomy and bilateral salpingo-oophorectomy. On laparotomy there was also incidental finding of ruptured mucin-filled appendix for which appendectomy was done. Histopathology examinations from all resected specimens revealed the diagnosed LAMN. Two weeks after surgical resection, she was started on FOLFOX chemotherapy regimen.
Discussion
Incidence of low grade appendiceal mucinous neoplasm is increasing. In addition to the increasing incidence, lack of early detection and impeded access to optimal multi-disciplinary treatment may worsen survival outcomes. Developing quality diagnostic services in the proper health context is crucial for early diagnosis and successful therapy of LAMN patients, and applying a resource-sensitive approach to prioritize essential treatments based on effectiveness and cost-effectiveness is key to overcoming barriers in low- and middle-income countries.
Conclusion
A recognition of mucinous material and abnormal appearing appendix should prompt the surgeon to consider performing an appendectomy to obtain primary pathologic diagnosis.
Keywords: Low grade appendiceal mucinous neoplasm, Pseudomyxoma peritonei, Low- and middle-income countries, Ovarian cancer, Case report
Highlights
-
•
Given the documented disguise of LAMN as primary ovarian cancer diagnosis and treatment should be understood.
-
•
Performing an appendectomy is advised to obtain primary pathologic diagnosis upon a recognition of mucinous material.
1. Introduction
Low-grade appendiceal mucinous neoplasms are unique tumors of the appendix, characterized by low-grade mucinous epithelium with villiform, undulating, or flat architecture. These tumors lack infiltrative growth or destructive invasion, but can extend into the appendiceal wall by a “pushing” pattern of invasion, with a broad front that can mimic a diverticulum. These neoplasms have a propensity for peritoneal dissemination, resulting in the clinical presentation of pseudomyxoma peritonei [1]. LAMN is an uncommon tumor of the appendix that is usually diagnosed incidentally after surgery. Although LAMN may be asymptomatic, it can rupture and seed mucin and neoplastic epithelium into the peritoneum PMP [2]. Approximately 20 % of appendiceal mucinous neoplasms spread peritoneally, leading to PMP, the presence of intraperitoneal mucin [3,4]. The clinical presentation of LAMN can be quite variable. Some patients are asymptomatic with the lesion incidentally identified during imaging or other operative procedures, while other patients may present with abdominal pain, weight loss, and acute appendicitis. In advanced disease, there may be the presence of pseudomyxoma peritonei, abdominal distension, and abdominal hernia [[5], [6], [7]].
Although preoperative diagnosis can be made by computed tomography examination [[8], [9], [10]], LAMN is mostly frequently identified intraoperatively or even postoperatively incidentally. This issue particularly concerns female patients, since metastatic ovarian mucinous neoplasms also share similar atypical clinical manifestations and imaging findings [11]. The most effective differential diagnostic technique known for LAMN is immunohistochemical examination. The most common immunohistochemistry markers applied for diagnostic purposes include cytokeratin 7, CK20, caudal type homeobox 2, paired box gene 8 and special AT-rich sequence-binding protein 2 [[12], [13], [14]].
When PMP develops, the condition and surgical treatment can lead to significant morbidity and mortality. Both LAMN and PMP can present as an abdominopelvic mass and be mistaken for an ovarian neoplasm, as primary ovarian malignancies often present with the same symptoms. Awareness of LAMN by gynecologic oncologists is warranted given this mimicry. Given the documented disguise of LAMN as primary ovarian cancer and increasing incidence of LAMN, diagnosis and treatment should be understood [15]. Here in, we report a case of LAMN presenting as abdominal swelling which was clinically considered as malignant ovarian tumor. We are reporting this case due to its rarity and to emphasize the importance of considering LAMP in the differential diagnosis of abdominal swelling for early diagnostic workup and management. This has been reported in line with SCARE criteria [16].
2. Case presentation
A 37-year-old patient presented with a complaint of abdominal swelling of six months duration which was initially small but progressively increased to attain the current size. She had visited a nearby health center on multiple occasions for this complaint and took unspecified medications, but no improvement. Associated with this she had a history of early satiety, bloating, night sweating, unquantified weight loss, easy fatigability and difficulty of defecation. There was no history of yellowish discoloration of the eye, cough, reddish discoloration of urine and difficulty of urination. All her previous deliveries were vaginally and it was uneventful. She had no history of diabetes, hypertension, or other chronic disease. Her past medical history is not significant. She had no previous history of admission to hospital. She had no history of any form of surgical procedures. On physical examination, there was mild abdominal tenderness, 20 weeks equivalent adnexal mass, pale conjunctiva and non-icteric sclera. On the basis of the above findings, a provisional clinical impression of ovarian tumor was entertained. Liver was not palpable below costal margin. There was no splenomegaly. Other clinical findings were within normal limits. Her blood group was .
Urine B- human chorionic gonadotropin was negative. Her serum cancer antigen 125 and carcinoembryonic antigen levels were 189 iu/ml and 10 ng/mL respectively. Abdominopelvic ultrasound was done and showed 20x10x10 cm right and 10x10x7cm left complex cystic ovarian masses having thick septa and was adherent to the uterus and urinary bladder. Due to limited number of Gynecologic Oncologists and long waiting list of patients, she underwent total abdominal hysterectomy, omentectomy, bilateral salpingo-oophorectomy and appendectomy after one week of her initial presentation. The intra-operation finding was huge cystic ovarian masses having perforation at posterior side with jelly like leakage to the peritoneal cavity. Peritoneum, bowel, omentum, spleen, liver and diaphragm were involved by tumor deposit.
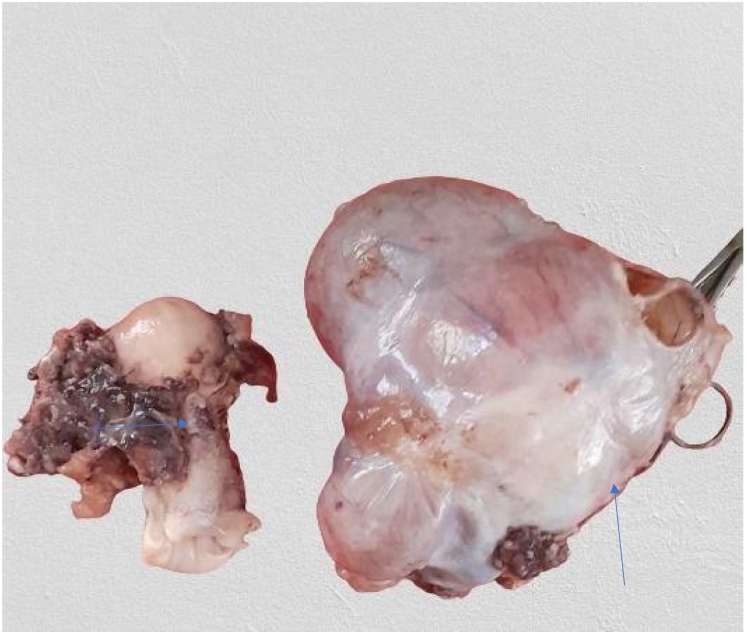
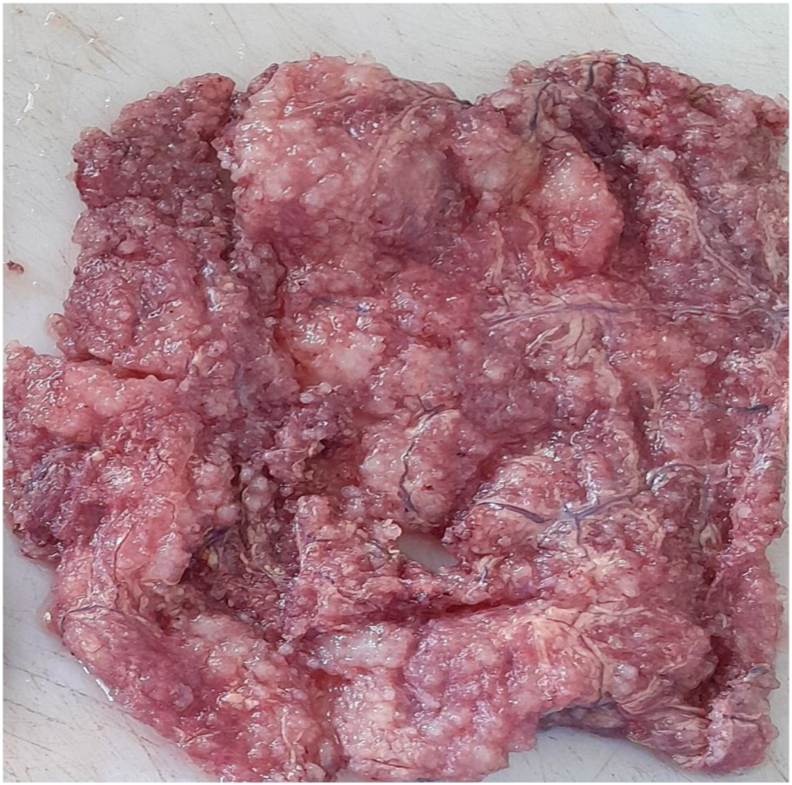
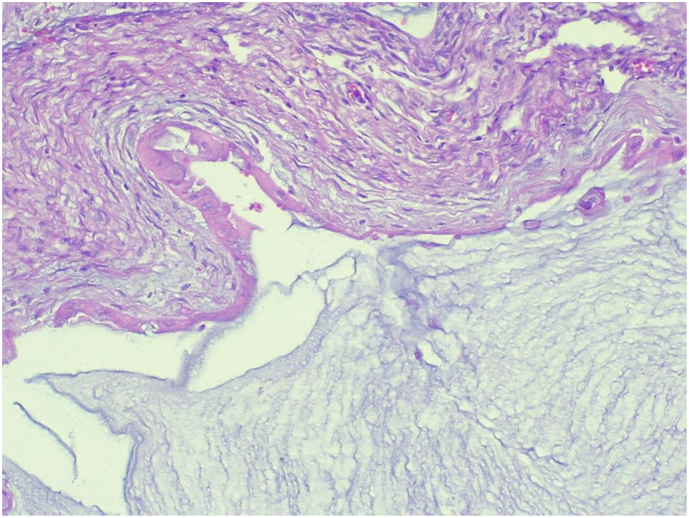
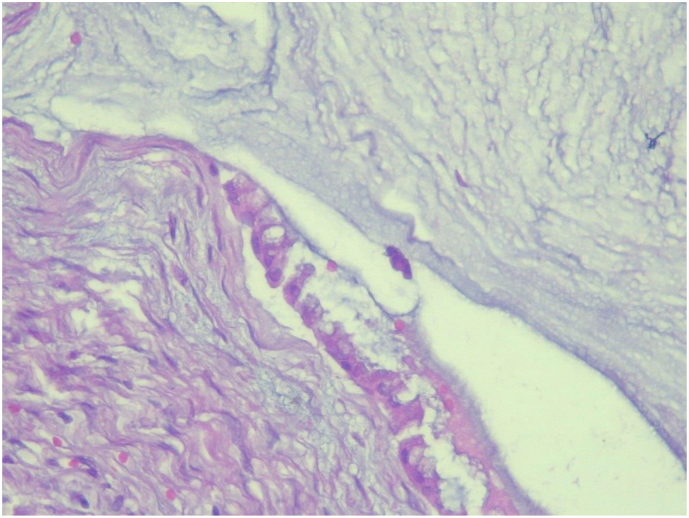
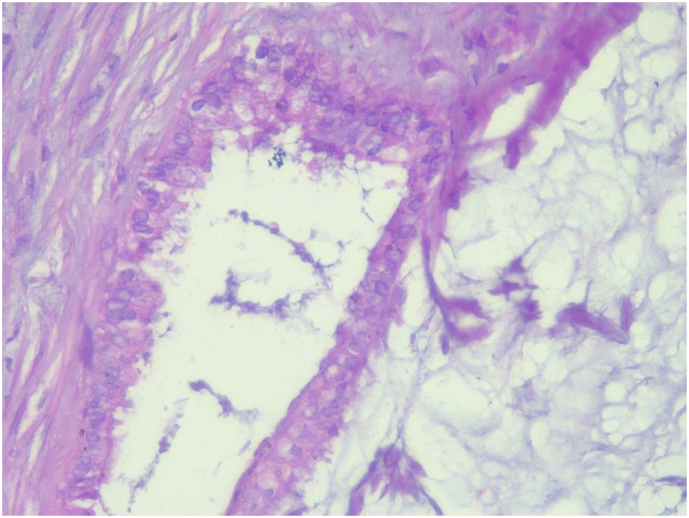
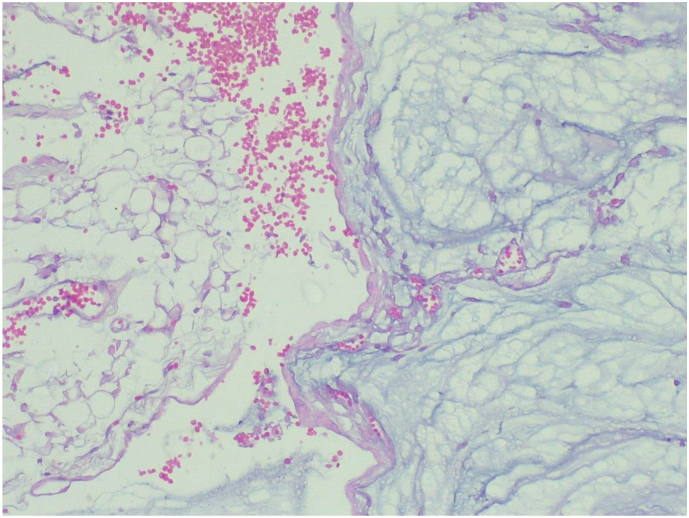
The specimen was sent to pathology department for gross and histopathologic examination. Gross examination of complex cystic ovarian masses revealed 20x10x10 cm right and 10x10x7cm left ovarian masses with smooth outer surface (Fig. 1, Fig. 2). Gross examination of omentectomy specimen revealed numerous glistening nodularities that have jelly like consistency (Fig. 3). Gross evaluation of the appendix revealed a 3.5 cm rupture and present at the proximal appendiceal resection margin (Fig. 4). In the setting of large ruptured primary tumor, LAMN may be tracking along the appendix. Cut surface examination of both ovarian masses showed multilocular cystic spaces filled with jelly like material (Fig. 5). Cut surface through uterus and cervix were unremarkable. Microscopic examinations from both ovarian masses, perforated appendix, omentum and peritoneum showed abundant mucin characteristically exhibiting expansile growth with a pushing border and/or slender villi lined by bland hypermucinous epithelium (Fig. 6, Fig. 7, Fig. 8, Fig. 9).
Fig. 1.
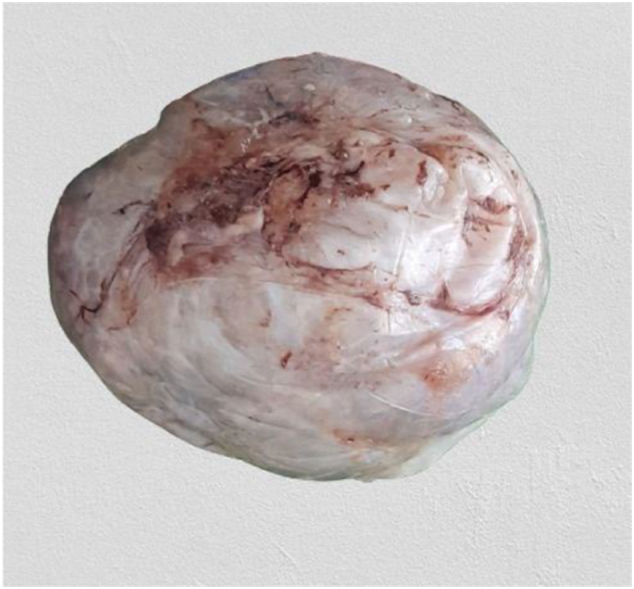
20x10x10 cm complex cystic right ovarian mass with smooth outer surface.
Fig. 2.
Total abdominal hysterectomy specimen (horizontal arrow) and 10x10x7cm complex cystic left ovarian mass with smooth outer surface (vertical arrow).
Fig. 3.
Omentectomy specimen showing numerous glistening nodularities.
Fig. 4.

Appendectomy specimen with a ruptured focus.
Fig. 5.

Cut surface examination of both ovarian masses showed multilocular cystic spaces filled with jelly like material.
Fig. 6.
Low power (10X) microscopic examination of complex cystic ovarian masses showing abundant mucin characteristically exhibiting expansile growth with a pushing border (Hematoxylin and Eosin stain).
Fig. 7.
High power (40X) microscopic examination of complex cystic ovarian masses showing abundant mucin characteristically exhibiting expansile growth with a pushing border (Hematoxylin and Eosin stain).
Fig. 8.
High power (40X) microscopic examination of appendectomy specimen showing abundant mucin characteristically exhibiting expansile growth with a pushing border (Hematoxylin and Eosin stain).
Fig. 9.
High power (40X) microscopic examination of omentectomy specimen showing abundant mucin characteristically exhibiting expansile growth with a pushing border (Hematoxylin and Eosin stain).
On the basis of above findings, the case was diagnosed as grade I, LAMN, P (T4b, NX, M1c). The patient was having good post-operative condition. Two weeks after surgical resection, she was started on FOLFOX (Oxaliplatin 85 mg/m2 IV, Leucovorin 400 mg/m2 IV, Fluorouracil 400 mg/m2 IV bolus then 2400 mg/m2 IV administered over 46 h) chemotherapy regimen every two weeks for 12 rounds. Since then, she was followed with regular serum CEA, complete blood count, organ function tests and abdominopelvic CT scan. The patient was having a smooth course with no significant adverse effects encountered. Currently the patient took her 4th round chemotherapy regimen and she was doing good.
3. Discussion
Preoperative differentiation between PMP versus metastatic ovarian cancer is difficult. Pathologically, LAMN describes a neoplasm with dysplastic epithelium that produces abundant mucin and characteristically exhibits expansile growth with a “pushing” border [15]. In this case, the postoperative appendiceal, ovarian, omental and peritoneal biopsy revealing slender villi lined by bland hypermucinous epithelium may have been an indicator of the diagnosis; however, in the absence of immunohistochemical examination the biopsy findings alone were not sufficient to differentiate ovarian from appendiceal origin. Although LAMN/PMP may present with presumed ovarian involvement, PMP rarely develops from ovarian neoplasia. If PMP is thought to be of ovarian origin, it may develop as a mucinous tumor within a mature teratoma [17].
Primary ovarian mucinous tumors can be difficult to distinguish from metastatic gastrointestinal neoplasms by histology alone. The expected immunoprofile of a suspected metastatic lower gastrointestinal tumor is CK7−/CK20+/CDX2+/PAX8−. The immunohistochemical profile of most “intestinal-type” primary ovarian mucinous primaries is distinct from lower gastrointestinal neoplasms. A combination of the immunohistochemical markers CK7 and SATB2 is highly sensitive and specific for distinguishing primary ovarian mucinous tumors from colorectal and appendiceal metastases. The typical immunohistochemical staining pattern for an ovarian mucinous carcinoma by using Hematoxylin and eosin stain is, CK7 diffuse, SATB2 absent, PAX8 focal, CDX2 focal and CK20 focal. The typical immunohistochemical staining pattern for low-grade appendiceal mucinous neoplasm by using H&E stain is, CK7 absent, SATB2 diffuse, PAX8 absent, CDX2 diffuse and CK20 diffuse. The typical immunohistochemical staining pattern for colorectal carcinoma by using H&E stain is, CK7 absent, SATB2 diffuse, PAX8 absent, CDX2 diffuse and CK20 diffuse [18].
Coming to our case, the diagnosis of LAMN was rendered by histopathology examination of resected specimens and we were unable to know its immunohistochemical staining pattern due to resource limited and poor set up country. Although there are few private sector clinical laboratories in our country which help clinicians in molecular diagnosis of cancer, majority of patients cannot afford the high cost of molecular studies. The same was true for our patient.
Metastasis from the appendix to the ovary might be more common to the right rather than left ovary, due to proximity [19]. Unilaterality and large size (>10 or > 13 cm) are gross features in keeping with a primary ovarian mucinous neoplasm, rather than a metastasis from the gastrointestinal tract [20,21]. See Table 1 for a summary of a proposed intraoperative assessment guide for gynecologic surgeons who suspect LAMN [15]. CT of the chest, abdomen, and pelvis is the most common imaging modality used to evaluate the primary tumor and assess for metastatic disease [22]. Magnetic resonance imaging can detect extraluminal mucin and has also been shown to be superior to CT in the detection of peritoneal disease using a combination of diffusion-weighted imaging and delayed post gadolinium sequences [23]. Coming to our case, preoperative CT scan examination was not done. Postoperative surveillance for localized and completely resected LAMN is to obtain MRI with tumor markers every 6 months for 2 years because most early recurrences occur within that timeframe [24]. In patients with acellular or low-grade peritoneal disease who have undergone cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, CT or MRI of the abdomen and pelvis is recommended at 2 months postoperatively (baseline), then annually for ≥5 years [25,26]. The same was true for our patient who was having baseline abdominopelvic CT scan examination at 2 months postoperatively.
Table 1.
Intraoperative assessment to distinguish between primary ovarian malignancy versus metastasis to ovary or appendiceal neoplasm.
| Features of likely primary ovarian neoplasm | Features of likely metastasis to ovary | Features of likely primary appendiceal neoplasm |
| Smooth capsule | Bilateral ovarian mass | Not involving ovaries |
| Evenly distributed cystic and solid areas without discrete nodularity | Nodular pattern | Gross mucinous multinodular appearance |
| Size >10–13 cm | Hilar involvement | Pseudomyxoma peritonei |
| Mural nodules | Colloid morphology | Intraperitoneal spread |
| Non-mucinous type on frozen section | Mucinous type on frozen section | Macroscopic appendiceal lesion or perforation |
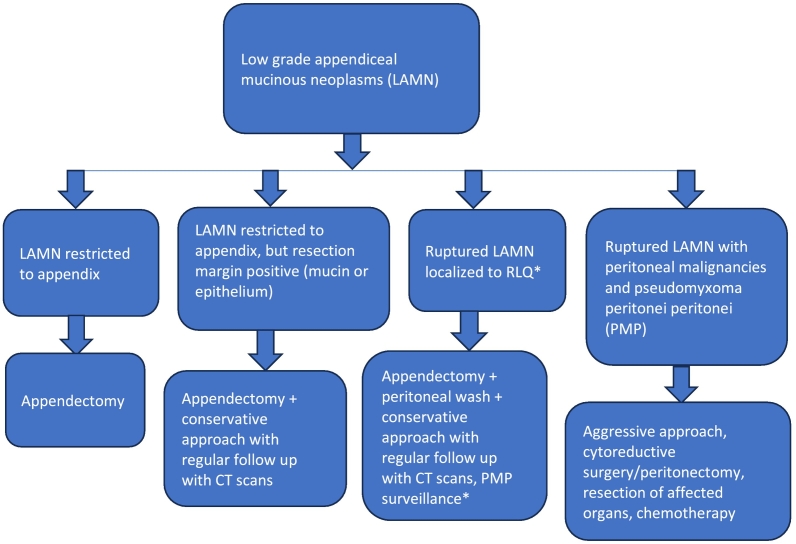
The serum tumor markers CEA, CA19–9, and CA125 are frequently obtained on diagnosis of appendiceal mucinous neoplasms and routinely monitored to assess disease remission or progression [27]. Coming to our case, her baseline serum CA 125 and CEA levels were 189 iu/ml and 10 ng/mL respectively. Because LAMN does not spread via the hematogenous or lymphatic route, an appendectomy is generally acceptable for the management of LAMN confined to the appendix on pathology, with conservative follow-up and surveillance if the appendectomy margins involve acellular mucin or neoplastic epithelium (Fig. 10) [4,28,29]. CRS and HIPEC have become standard of care for many patients with metastatic epithelial appendiceal cancer [30]. Coming to our case, the patient was started on FOLFOX chemotherapy regimen since HIPEC was not available in our set up.
Fig. 10.
Algorithm for management of LAMN.
*No consensus for ruptured LAMN with malignant cells in right lower quadrant – most takes a conservative approach.
Our LAMN case report signifies since both LAMN and PMP can present as an abdominopelvic mass and be mistaken for an ovarian tumor, awareness of LAMN by gynecologic oncologists is warranted given this mimicry and the documented disguise of LAMN as primary ovarian tumor and increasing incidence of LAMN, diagnosis and treatment should be understood. In order to maximize the benefits of cancer prevention programs, it is worth identifying and defining investment opportunities in the healthcare system, with clinical research collaborations between high income countries and LMICs being a helpful strategy to improve health indicators and prevent the burnout of health workers. The patient was very satisfied with the intervention and care given. 4.
4. Conclusion
This case is an example of why LAMN should be within the differential diagnosis of gynecologic surgeons when presented with a patient with bilateral ovarian masses and biopsy of acellular mucin. Intraoperatively, an abnormal appearing appendix with normal appearing gynecologic structures should trigger suspicion for appendiceal rather than ovarian origin. Intraoperatively, a recognition of mucinous material and abnormal appearing appendix should prompt the surgeon to consider performing an appendectomy to obtain primary pathologic diagnosis. A high level of suspicion could better optimize the patient for a joint case with the appropriate surgeons. Given the documented disguise of LAMN as primary ovarian cancer and increasing incidence of LAMN, diagnosis and treatment should be understood.
Ethics approval and consent to participate
Written informed consent for participation was obtained from the patient. We have also obtained ethical approval regarding the case from School of Medicine Ethical Review Board. A copy of the consent form as well as ethical approval is available for review by the Editor of this journal.
Guarantor
Endeshaw Asaye Kindie
Research registration number
Research registry 10605
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
N/A
Author contribution
Endeshaw Asaye Kindie performed the gross and histopathologic examination of the total abdominal hysterectomy, omentectomy, bilateral salpingo-oophorectomy and appendectomy specimen, and were major contributors in writing the case report. Girma Damtew Addisu, Eyoel Negash Taddesse, Nigat Amsalu Addis and Getachew Shiferaw Yigzaw revised the case report. All authors read and approved the case report.
Declaration of competing interest
N/A
Acknowledgements
N/A
Contributor Information
Endeshaw Asaye Kindie, Email: endeshaw.asaye@gmail.com.
Nigat Amsalu Addis, Email: Nigat.Amsalu@uog.edu.et.
Data availability
Data sharing does not apply to this article as no new data were created or analyzed in this study.
References
- 1.Umetsu S.E., Kakar S. Staging of appendiceal mucinous neoplasms: challenges and recent updates. Hum. Pathol. 2023 Feb 1;132:65–76. doi: 10.1016/j.humpath.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Guner M., Aydın C. Low-grade appendiceal mucinous neoplasm: what is the best treatment? Cureus. 2023 Oct;15(10) doi: 10.7759/cureus.46591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiter Shelby, et al. Progression to pseudomyxoma peritonei in patients with low grade appendiceal mucinous neoplasms discovered at time of appendectomy. Am. J. Surg. 2022;223(6):1183–1186. doi: 10.1016/j.amjsurg.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Misdraji J. Mucinous epithelial neoplasms of the appendix and pseudomyxoma peritonei. Mod. Pathol. 2015 Jan 1;28:S67–S79. doi: 10.1038/modpathol.2014.129. [DOI] [PubMed] [Google Scholar]
- 5.Tirumani S.H., Fraser-Hill M., Auer R., Shabana W., Walsh C., Lee F., Ryan J.G. Mucinous neoplasms of the appendix: a current comprehensive clinicopathologic and imaging review. Cancer Imaging. 2013;13(1):14. doi: 10.1102/1470-7330.2013.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-Moreno S., Sugarbaker P.H. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Journal of British Surgery. 2004 Mar;91(3):304–311. doi: 10.1002/bjs.4393. [DOI] [PubMed] [Google Scholar]
- 7.Dixit A., Robertson J.H., Mudan S.S., Akle C. Appendiceal mucocoeles and pseudomyxoma peritonei. World J Gastroenterol: WJG. 2007 Apr 4;13(16):2381. doi: 10.3748/wjg.v13.i16.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arshi J., Peter A.D., Liang Y., Hao Y. 38(1) 2024 Jan 1. Confined Low Grade Appendiceal Mucinous Neoplasm with Coexisting Distant Metastasis. in vivo; pp. 295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzmán G.A., Montealegre I., Obando A.M. Incidental diagnosis of a low-grade mucinous appendicular neoplasm: a case report. Int. J. Surg. Case Rep. 2021 Jun 1;83 doi: 10.1016/j.ijscr.2021.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X.R., Mao J., Tang W., Meng X.Y., Tian Y., Du Z.L. Low-grade appendiceal mucinous neoplasms confined to the appendix: clinical manifestations and CT findings. J. Invest. Med. 2020 Jan;68(1):75–81. doi: 10.1136/jim-2018-000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perivoliotis K., Christodoulidis G., Samara A.A., Sgantzou I.K., Floros T., Volakakis G., Karasavvidou F., Tepetes K. Case Reports in Surgery 2021. Vol. 1. 2021. Low-Grade Appendiceal Mucinous Neoplasm (LAMN) Primarily Diagnosed as an Ovarian Mucinous Tumor; p. 5523736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmoeckel E., Kirchner T., Mayr D. SATB2 is a supportive marker for the differentiation of a primary mucinous tumor of the ovary and an ovarian metastasis of a low-grade appendiceal mucinous neoplasm (LAMN): a series of seven cases. Pathology-Research and Practice. 2018 Mar 1;214(3):426–430. doi: 10.1016/j.prp.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Aldaoud N., Erashdi M., AlKhatib S., Abdo N., Al-Mohtaseb A., Graboski-Bauer A. The utility of PAX8 and SATB2 immunohistochemical stains in distinguishing ovarian mucinous neoplasms from colonic and appendiceal mucinous neoplasm. BMC Research Notes. 2019 Dec;12 doi: 10.1186/s13104-019-4816-9. 1–6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borges A.L., Reis-de-Carvalho C., Chorão M., Pereira H., Djokovic D. Low-grade mucinous appendiceal neoplasm mimicking an ovarian lesion: a case report and review of literature. World J. Clin. Cases. 2021 Apr 4;9(10):2334. doi: 10.12998/wjcc.v9.i10.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen C.G., Hamid A., Chen A., Sood D., Jou J. Low grade appendiceal mucinous neoplasm metastatic to the ovary: a case report and intraoperative assessment guide. Int. J. Surg. Case Rep. 2023 Aug 1;109 doi: 10.1016/j.ijscr.2023.108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. Lond. Engl. 2023;109(5):1136. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKenney J.K., Soslow R.A., Longacre T.A. Ovarian mature teratomas with mucinous epithelial neoplasms: morphologic heterogeneity and association with pseudo- myxoma peritonei. Am. J. Surg. Pathol. 2008 May 1;32(5):645–655. doi: 10.1097/PAS.0b013e31815b486d. [DOI] [PubMed] [Google Scholar]
- 18.Meagher N.S., Wang L., Rambau P.F., Intermaggio M.P., Huntsman D.G., Wilkens L.R., El-Bahrawy M.A., Ness R.B., Odunsi K., Steed H., Herpel E. A combination of the immunohistochemical markers CK7 and SATB2 is highly sensitive and specific for distinguishing primary ovarian mucinous tumors from colorectal and appendiceal metastases. Mod. Pathol. 2019 Dec;32(12):1834–1846. doi: 10.1038/s41379-019-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RH Y. Mucinous tumor of appendix associated with mucinous tumors of the ovary and pseudomyxoma peritonei. A clinicopathological analysis of 22 cases supporting an origin in the appendix. Am. J. Surg. Pathol. 1991;15(5):415–429. doi: 10.1097/00000478-199105000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Hu J., Khalifa R.D., Roma A.A., Fadare O. The pathologic distinction of primary and metastatic mucinous tumors involving the ovary: a re-evaluation of algorithms based on gross features. Ann. Diagn. Pathol. 2018 Dec 1;37:1–6. doi: 10.1016/j.anndiagpath.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Dundr P., Singh N., Nožičková B., Němejcová K., Bártů M., Stružinská I. Primary mucinous ovarian tumors vs. ovarian metastases from gastrointestinal tract, pancreas and biliary tree: a review of current problematics. Diagn. Pathol. 2021 Dec;16:1–7. doi: 10.1186/s13000-021-01079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohani P., Scotti S.D., Shen P., et al. Use of FDG-PET imaging for patients with disseminated cancer of the appendix. Am. Surg. 2010;76:1338–1344. [PubMed] [Google Scholar]
- 23.Low R.N., Barone R.M., Lucero J. Comparison of MRI and CT for predicting the peritoneal Cancer index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann. Surg. Oncol. 2015;22:1708–1715. doi: 10.1245/s10434-014-4041-7. [DOI] [PubMed] [Google Scholar]
- 24.Guaglio M., Sinukumar S., Kusamura S., et al. Clinical surveillance after macroscopically complete surgery for low-grade appendiceal mucinous neoplasms (LAMN) with or without limited peritoneal spread: long-term results in a prospective series. Ann. Surg. Oncol. 2018;25:878–884. doi: 10.1245/s10434-018-6341-9. [DOI] [PubMed] [Google Scholar]
- 25.Roxburgh C.S., Fenig Y.M., Cercek A., et al. Outcomes of low-grade appendiceal mucinous neoplasms with remote acellular mucinous peritoneal deposits. Ann. Surg. Oncol. 2019;26(118):124. doi: 10.1245/s10434-018-7003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Hooser A., Williams T.R., Myers D.T. Mucinous appendiceal neoplasms: pathologic classification, clinical implications, imaging spectrum and mimics. Abdom. Radiol. (NY) 2018;43:2913–2922.8. doi: 10.1007/s00261-018-1561-9. [DOI] [PubMed] [Google Scholar]
- 27.Munoz-Zuluaga C.A., Sardi A., MacDonald R., et al. The role of preoperative tumor markers in patients with peritoneal carcinomatosis from appendiceal cancer undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2018;25 S155–S6. [Google Scholar]
- 28.Arnason T., Kamionek M., Yang M., Yantiss R.K., Misdraji J. Significance of proximal margin involvement in low-grade appendiceal mucinous neoplasms. Arch. Pathol. Lab. Med. 2015;139:518–521. doi: 10.5858/arpa.2014-0246-OA. [DOI] [PubMed] [Google Scholar]
- 29.Tiselius C., Kindler C., Shetye J., Letocha H., Smedh K. Computed tomography follow-up assessment of patients with low-grade appendiceal mucinous neoplasms: evaluation of risk for pseudomyxoma peritonei. Ann. Surg. Oncol. 2017;24:1778–1782. doi: 10.1245/s10434-016-5623-3. [DOI] [PubMed] [Google Scholar]
- 30.Hoehn R.S., Rieser C.J., Choudry M.H., Melnitchouk N., Hechtman J., Bahary N. Current management of appendiceal neoplasms. Am. Soc. Clin. Oncol. Educ. Book. 2021 Mar 25;41:118–132. doi: 10.1200/EDBK_321009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no new data were created or analyzed in this study.