Abstract
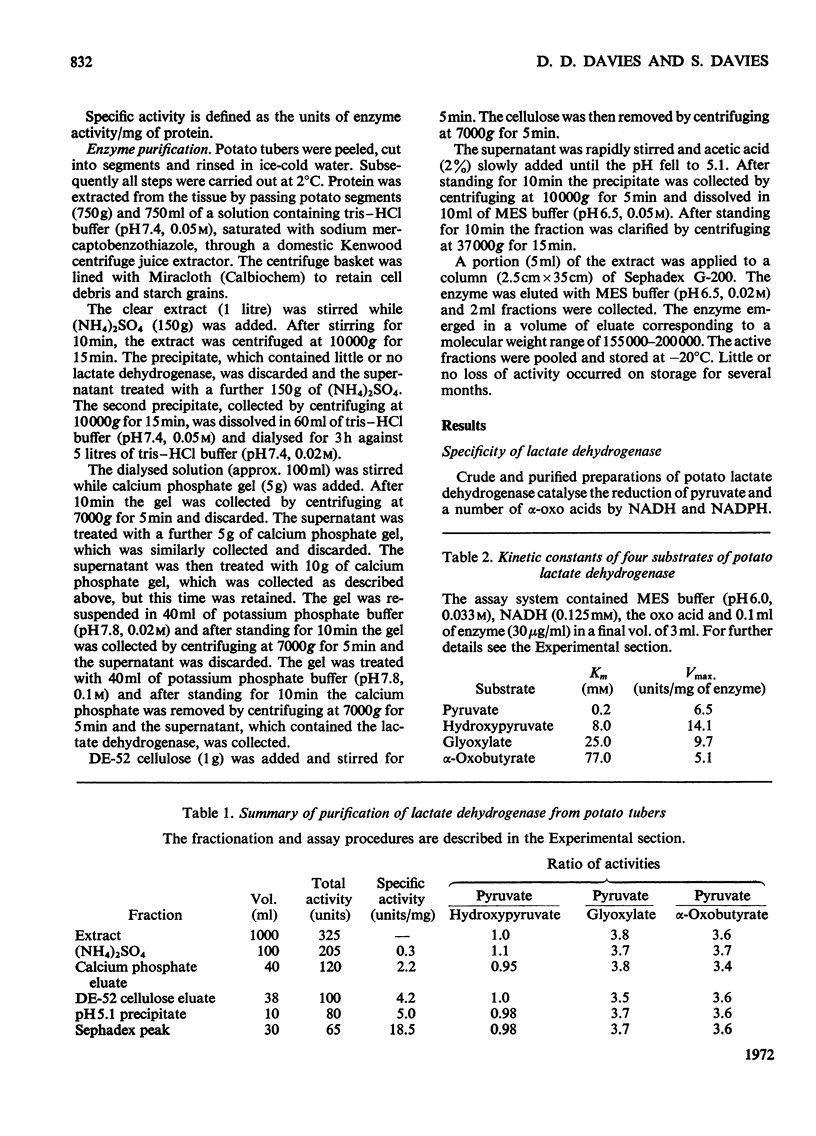
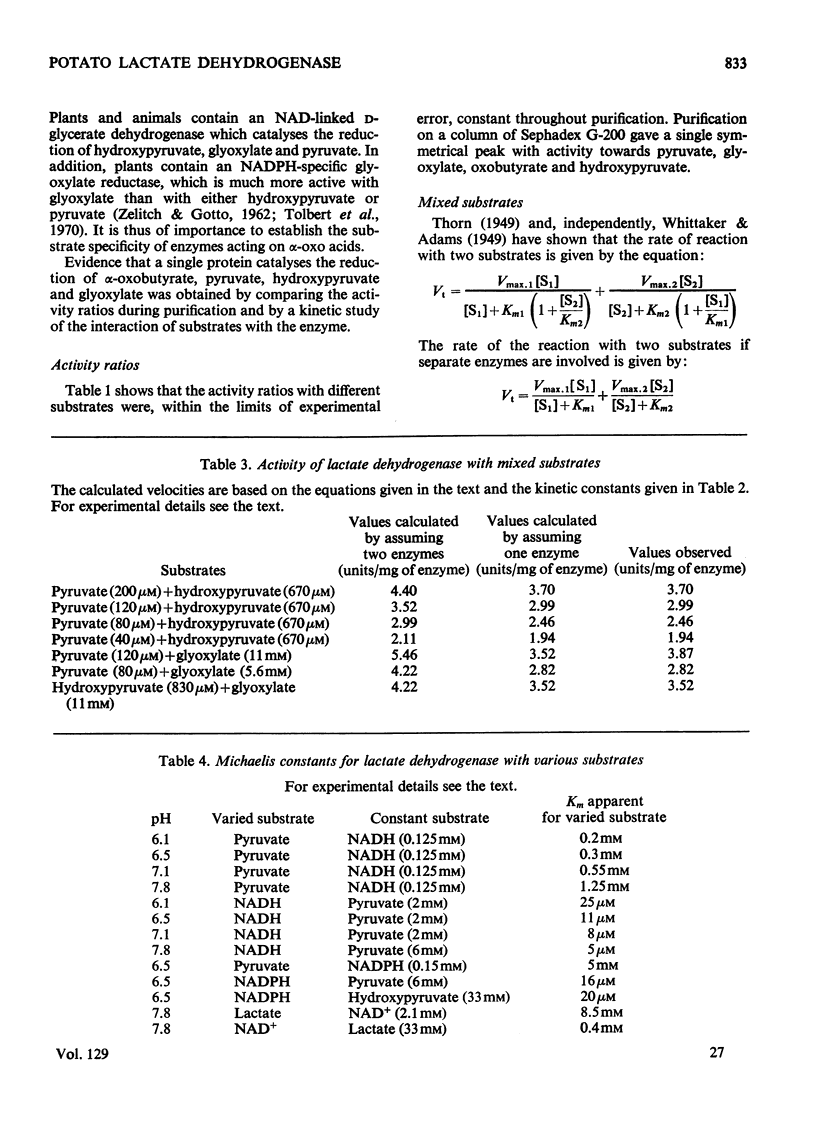
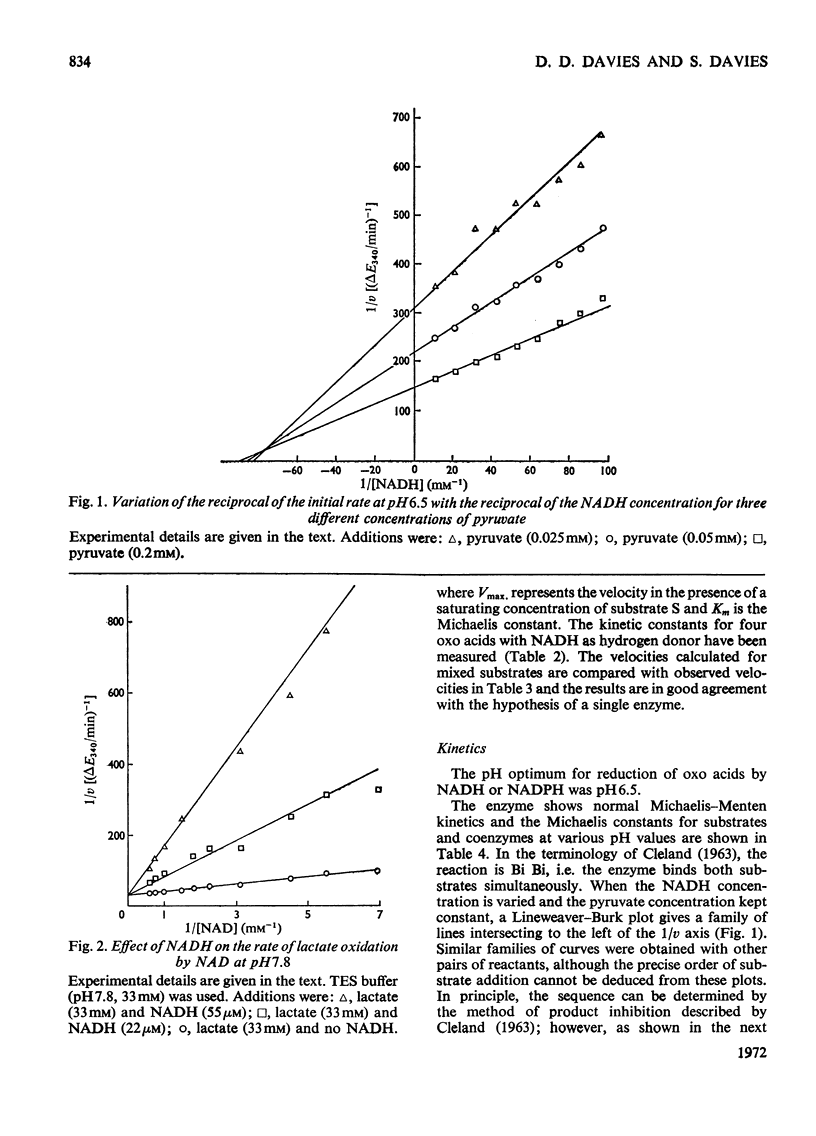
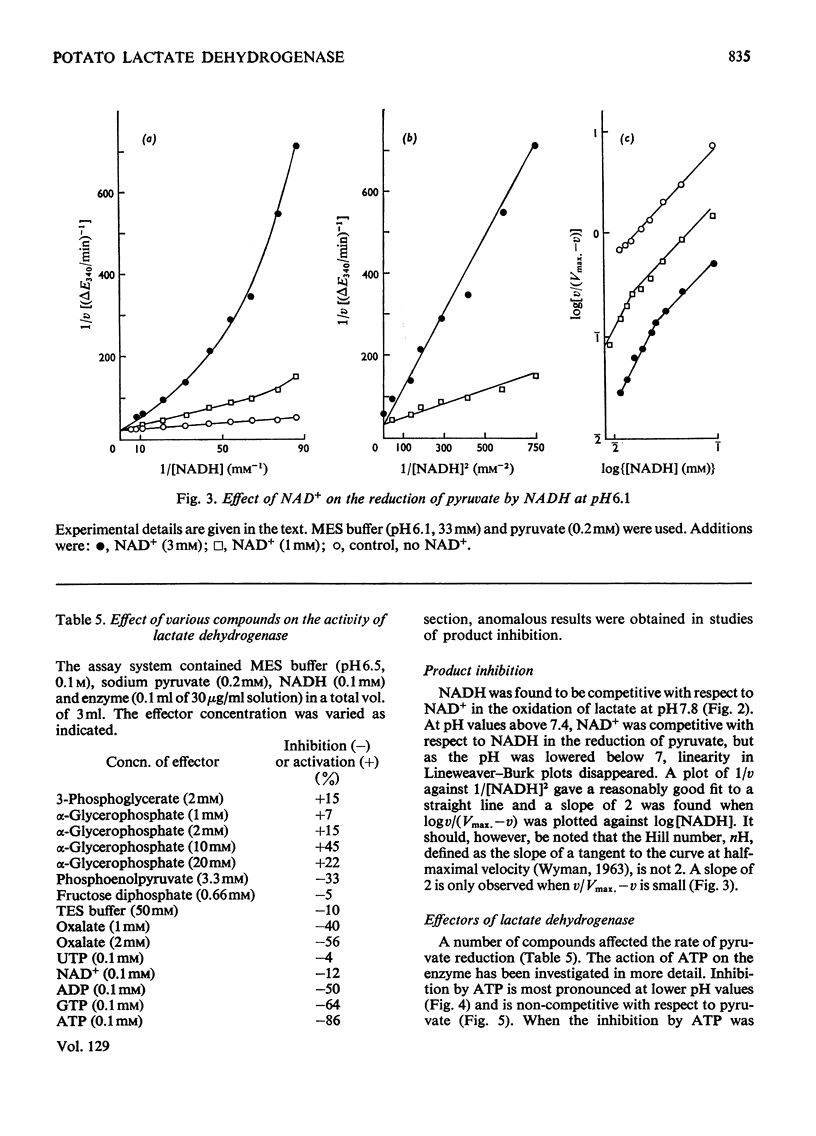
1. A purification of l(+)-lactate dehydrogenase is described. 2. The final preparation is active with NADH and NADPH and with a number of keto acids, but evidence is presented to support the view that a single enzyme is involved. 3. NAD+ showed product inhibition, but at slightly acid pH values there was evidence of co-operative binding. 4. At acid pH values ATP was a potent inhibitor and appears to be an allosteric effector. At neutral or alkaline pH values ATP behaved as a weak competitive inhibitor. 5. The physiological significance of inhibition by ATP is discussed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKER J., EL SAIFI A. F. Studies in the respiratory and carbohydrate metabolism of plant tissues. I. Experimental studies of the formation of carbon dioxide, lactic acid and other products in potato tubers under anaerobic conditions. Proc R Soc Lond B Biol Sci. 1952 Nov 20;140(900):362–385. doi: 10.1098/rspb.1952.0067. [DOI] [PubMed] [Google Scholar]
- BARRON E. S. G., LINK G. K. K., KLEIN R. M., MICHEL B. E. The metabolism of potato slices. Arch Biochem. 1950 Oct;28(3):377–398. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Kaloustian H. D., Kaplan N. O. Lactate dehydrogenase of lobster (Homarus americanus) tail muscle. II. Kinetics and regulatory properties. J Biol Chem. 1969 Jun 10;244(11):2902–2910. [PubMed] [Google Scholar]
- LOEWUS F. A., STAFFORD H. A. The enzymatic transfer of hydrogen by glyceric and lactic dehydrogenases. J Biol Chem. 1960 Nov;235:3317–3321. [PubMed] [Google Scholar]
- LéJohn H. B. D(-)-lactate dehydrogenases in fungi. Kinetics and allosteric inhibition by guanosine triphosphate. J Biol Chem. 1971 Apr 10;246(7):2116–2126. [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Chemical characterization of D-lactate dehydrogenase from Escherichia coli B. J Biol Chem. 1968 May 25;243(10):2579–2586. [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Wittenberger C. L. Kinetic studies on the inhibition of a (D(-)-specific lactate dehydrogenase by adenosine triphosphate. J Biol Chem. 1968 Jun 10;243(11):3067–3075. [PubMed] [Google Scholar]
- ZELITCH I., GOTTO A. M. Properties of a new glyoxylate reductase from leaves. Biochem J. 1962 Sep;84:541–546. doi: 10.1042/bj0840541. [DOI] [PMC free article] [PubMed] [Google Scholar]


