Abstract
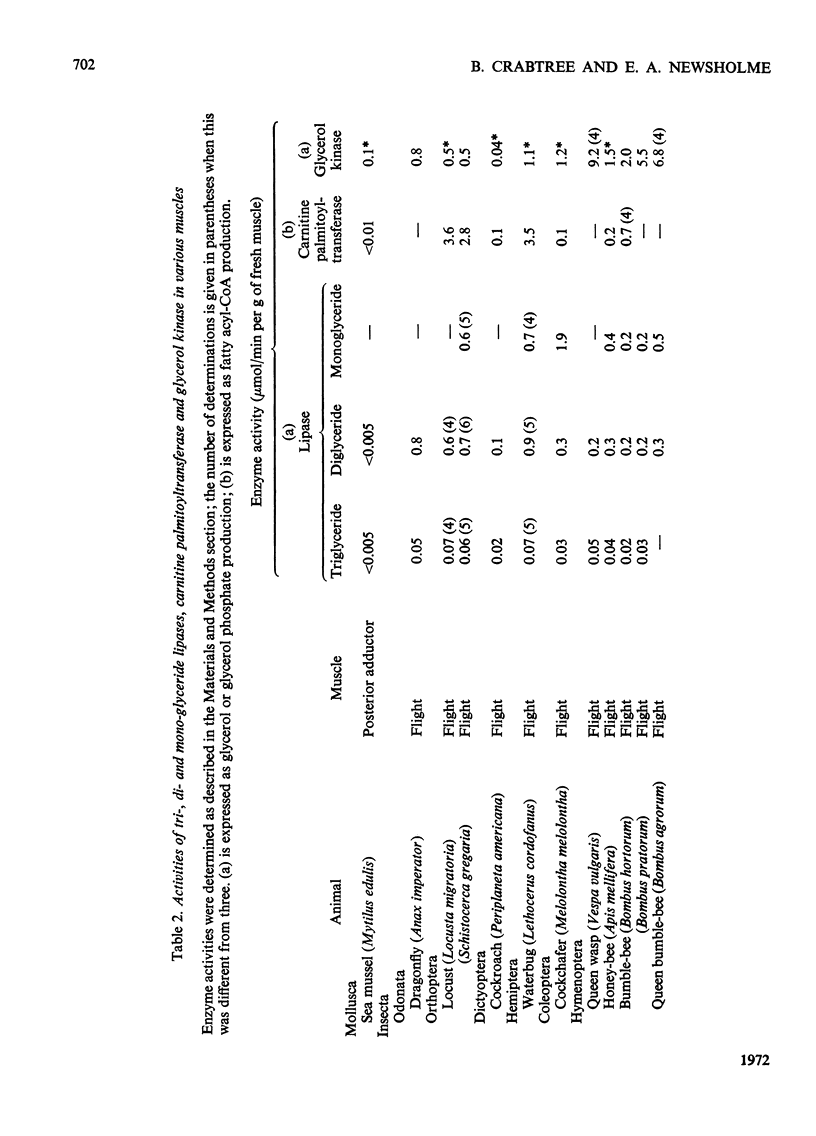
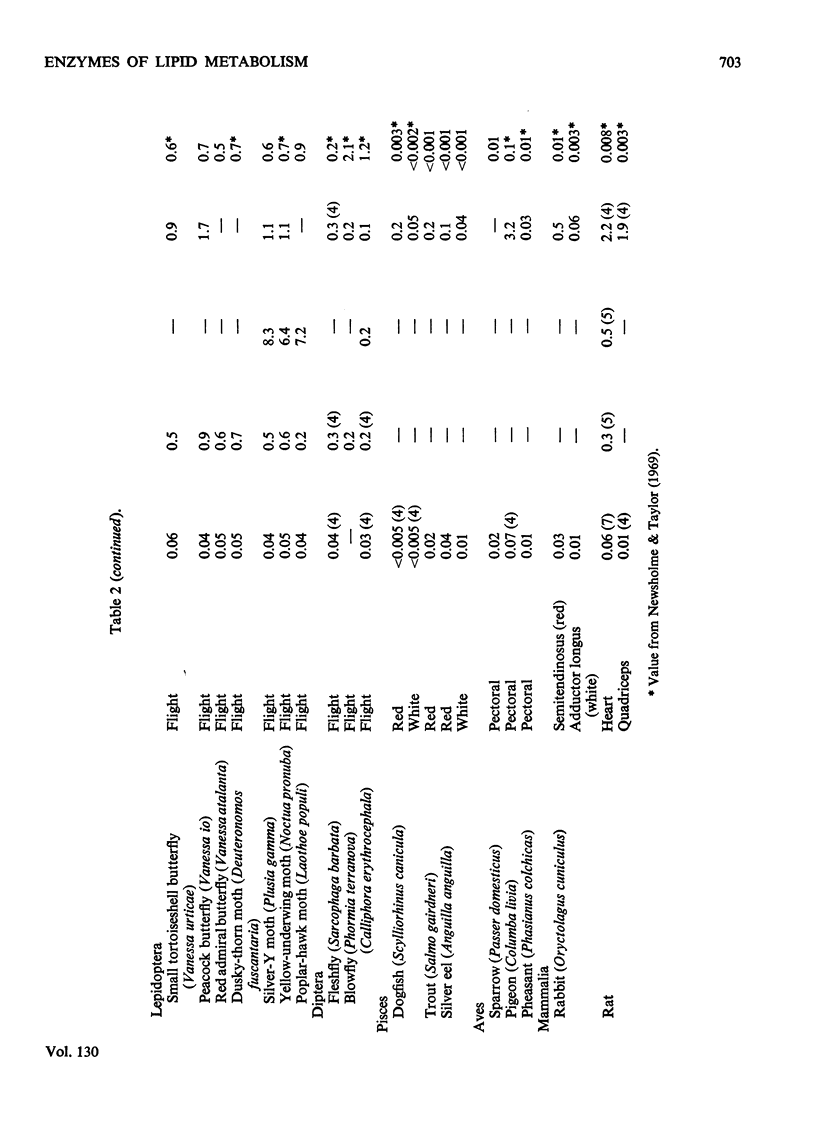
1. The activities of tri-, di- and mono-glyceride lipase and carnitine palmitoyltransferase were measured in homogenates of a variety of muscles. These activities were used to estimate the rate of utilization of glycerides and fatty acids by muscle. In muscles whose estimated rates of fat utilization can be compared with rates calculated for the intact muscle from such information as O2 uptake, there is reasonable agreement between the estimated and calculated rates. 2. In all muscles investigated the maximum rates of hydrolysis of glycerides increase in the order triglyceride, diglyceride, monoglyceride. The activity of diglyceride lipase is highest in the flight muscles of insects such as the locust, waterbug and some moths and is lowest in the flight muscles of flies, bees and the wasp. These results are consistent with the utilization of diglyceride as a fuel for some insect flight muscles. 3. In many muscles from both vertebrates and invertebrates the activity of glycerol kinase is similar to that of lipase. It is concluded that in these muscles the metabolic role of glycerol kinase is the removal of glycerol produced during lipolysis. However, in some insect flight muscles the activity of glycerol kinase is much greater than that of lipase, which suggests a different role for glycerol kinase in these muscles.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILINSKI E. Utilizatimn lipids by fish. I. Fatty acid oxidation by tissue slices from dark and white muscle of rainbow trout (Salmo gairdnerii). Can J Biochem Physiol. 1963 Jan;41:107–112. [PubMed] [Google Scholar]
- BJORNTORP P., FURMAN R. H. Lipolytic activity in rat heart. Am J Physiol. 1962 Aug;203:323–326. doi: 10.1152/ajplegacy.1962.203.2.323. [DOI] [PubMed] [Google Scholar]
- BREMER J. Carnitine in intermediary metabolism. The metabolism of fatty acid esters of carnitine by mitochondria. J Biol Chem. 1962 Dec;237:3628–3632. [PubMed] [Google Scholar]
- Beenakkers A. M. Carbohydrate and fat as a fuel for insect flight. A comparative study. J Insect Physiol. 1969 Mar;15(3):353–361. doi: 10.1016/0022-1910(69)90281-9. [DOI] [PubMed] [Google Scholar]
- Beenakkers A. M., Dewaide J. H., Henderson P. T., Lutgerhorst A. Fatty acid oxidation and some participating enzymes in animal organs. Comp Biochem Physiol. 1967 Sep;22(3):675–682. doi: 10.1016/0010-406x(67)90761-x. [DOI] [PubMed] [Google Scholar]
- Biale Y., Gorin E., Shafrir E. Characterization of tissue lipolytic and esterolytic activities cleaving full and partial glycerides. Biochim Biophys Acta. 1968 Jan 10;152(1):28–32. doi: 10.1016/0005-2760(68)90005-2. [DOI] [PubMed] [Google Scholar]
- CARROLL K. K. Separation of lipid classes by chromatography on Florisil. J Lipid Res. 1961 Apr;2:135–141. [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M., Walsh D. A., Krebs E. G. Activation of adipose tissue lipase by skeletal muscle cyclic adenosine 3',5'- monophosphate-stimulated protein kinase. J Biol Chem. 1970 Sep 25;245(18):4849–4851. [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochem J. 1972 Jan;126(1):49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crass M. F., 3rd, McCaskill E. S., Shipp J. C. Effect of pressure development on glucose and palmitate metabolism in perfused heart. Am J Physiol. 1969 Jun;216(6):1569–1576. doi: 10.1152/ajplegacy.1969.216.6.1569. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J. Concentrations of glycerides and phospholipids in rat heart and gastrocnemius muscles. Effects of alloxan-diabetes and perfusion. Biochem J. 1967 Aug;104(2):416–422. doi: 10.1042/bj1040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greville G. D., Tubbs P. K. The catabolism of long chain fatty acids in mammalian tissues. Essays Biochem. 1968;4:155–212. [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic synthesis of the coenzyme A derivatives of long chain fatty acids. J Biol Chem. 1953 Sep;204(1):329–343. [PubMed] [Google Scholar]
- KREBS H. A. RENAL GLUCONEOGENESIS. Adv Enzyme Regul. 1963;1:385–400. doi: 10.1016/0065-2571(63)90034-7. [DOI] [PubMed] [Google Scholar]
- LAWRIE R. A. The activity of the cytochrome system in muscle and its relation to myoglobin. Biochem J. 1953 Sep;55(2):298–305. doi: 10.1042/bj0550298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPMANN F., TUTTLE L. C. Lipase-catalysed condensation of fatty acids with hydroxylamine. Biochim Biophys Acta. 1950 Jan;4(1-3):301–309. doi: 10.1016/0006-3002(50)90036-9. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Robinson J., Taylor K. A radiochemical enzymatic activity assay for glycerol kinase and hexokinase. Biochim Biophys Acta. 1967 Mar 15;132(2):338–346. doi: 10.1016/0005-2744(67)90153-2. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Taylor K. A new principle for the assay of metabolites involving the combined effects of isotope dilution and enzymatic catalysis. Biochim Biophys Acta. 1968 Apr 16;158(1):11–24. doi: 10.1016/0304-4165(68)90067-6. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Taylor K. Glycerol kinase activities in muscles from vertebrates and invertebrates. Biochem J. 1969 May;112(4):465–474. doi: 10.1042/bj1120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., Garland P. B., Hales C. N., Newsholme E. A., Denton R. M., Pogson C. I. Interactions of metabolism and the physiological role of insulin. Recent Prog Horm Res. 1966;22:1–48. doi: 10.1016/b978-1-4831-9825-5.50004-x. [DOI] [PubMed] [Google Scholar]
- Stevenson E. The carnitine-independent oxidation of palmitate plus malate by moth flight-muscle mitochondria. Biochem J. 1968 Nov;110(1):105–110. doi: 10.1042/bj1100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz A. Fat transport in the locust: the role of diglycerides. Eur J Biochem. 1967 Sep;2(2):236–242. doi: 10.1111/j.1432-1033.1967.tb00130.x. [DOI] [PubMed] [Google Scholar]
- WIGGLESWORTH V. B. The utilization of reserve substances in Drosophila during flight. J Exp Biol. 1949 Aug;26(2):150-63, illust. doi: 10.1242/jeb.26.2.150. [DOI] [PubMed] [Google Scholar]


