Abstract
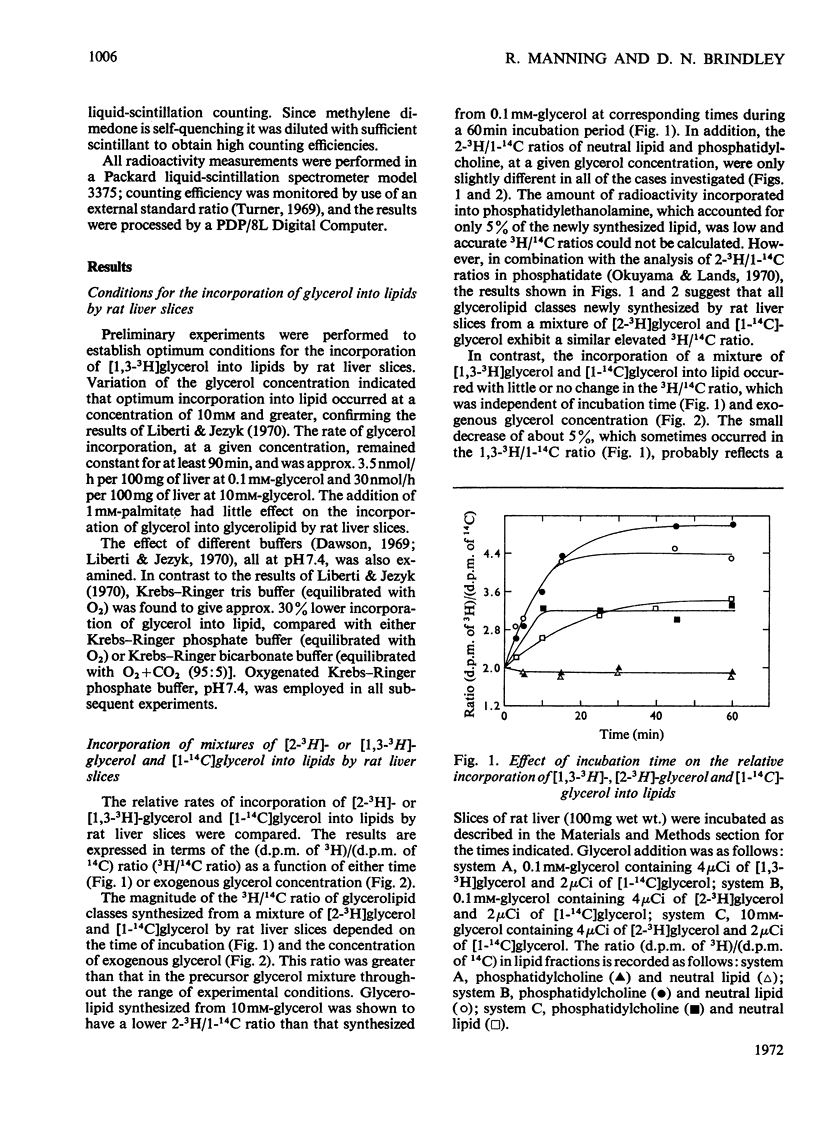
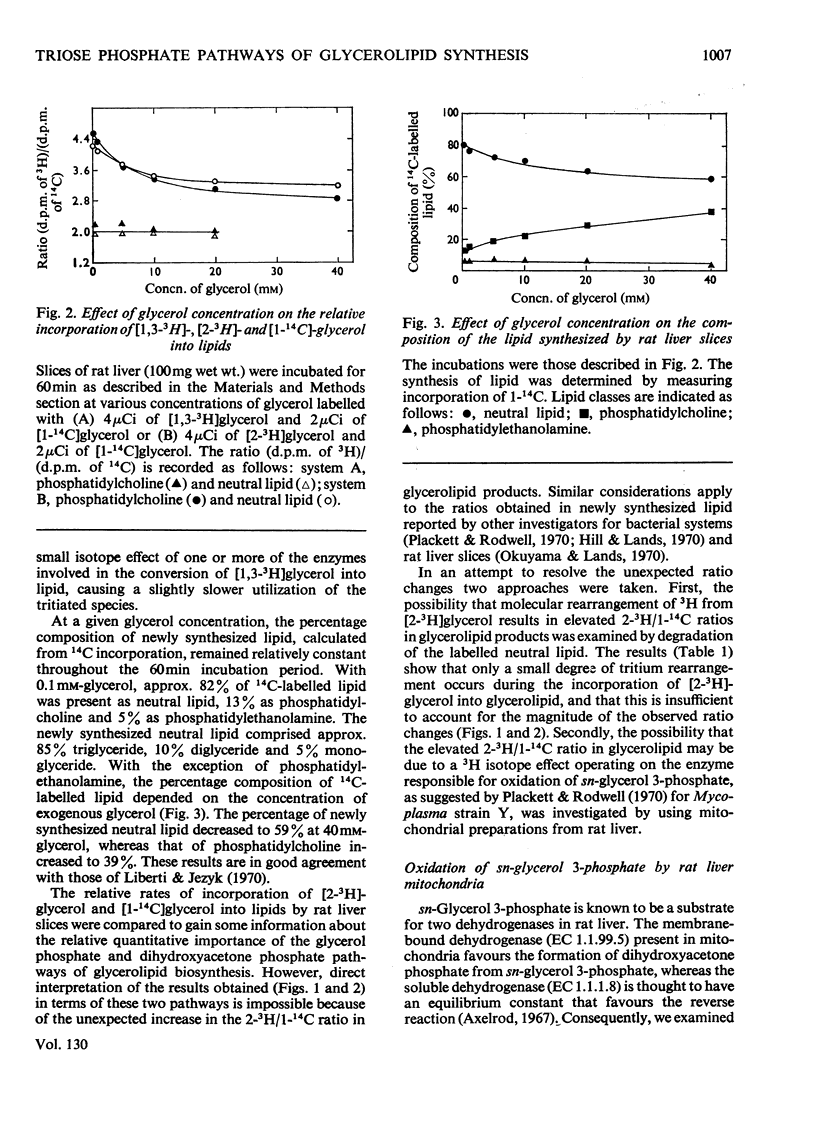
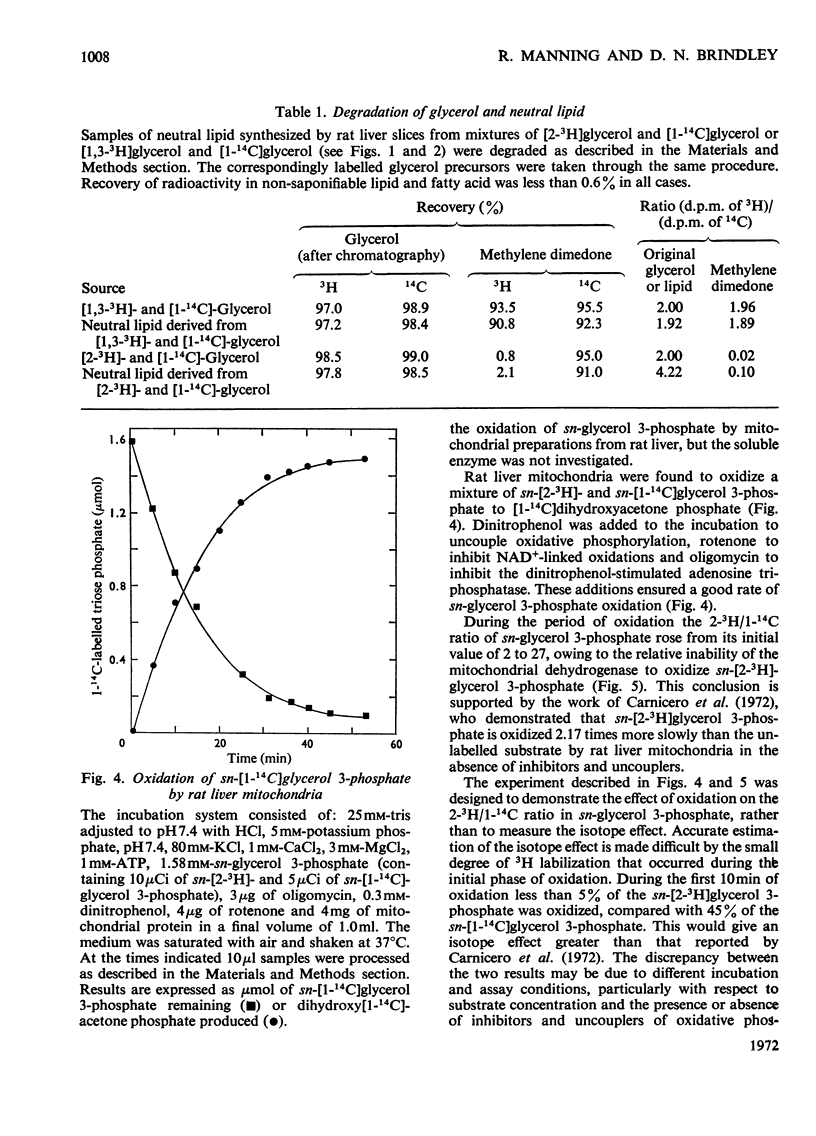
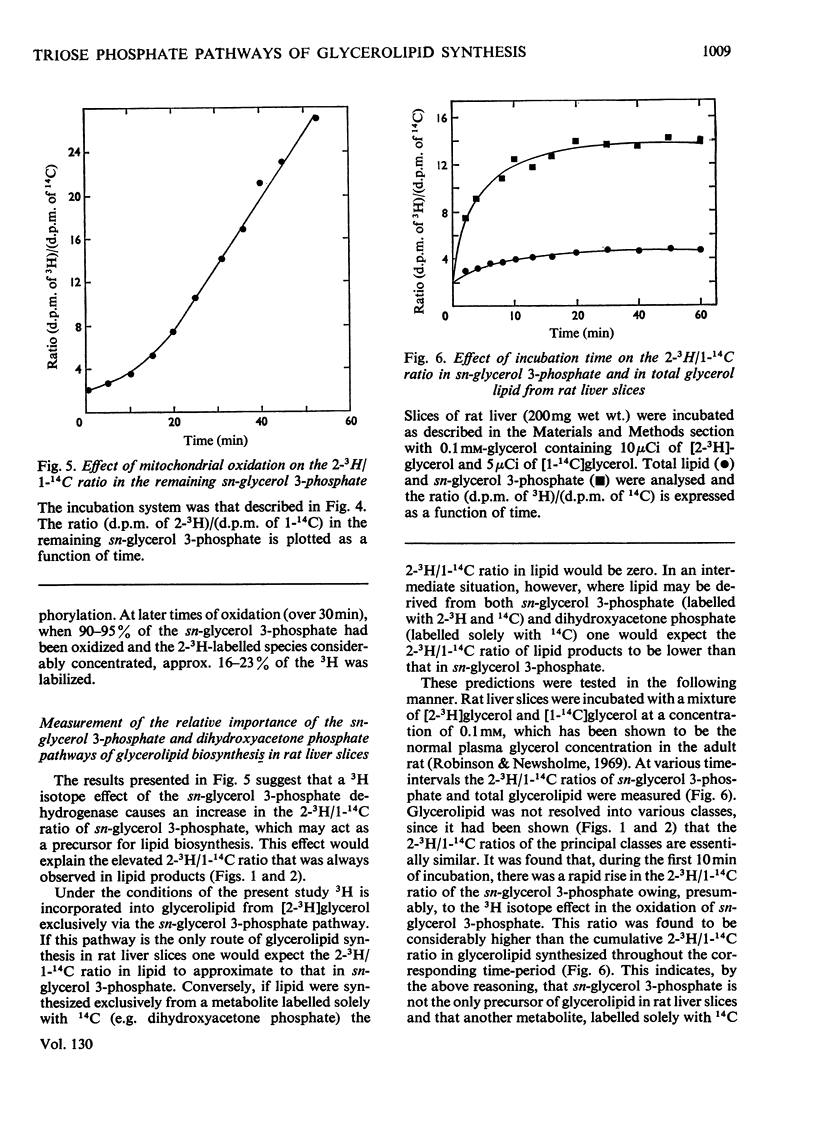
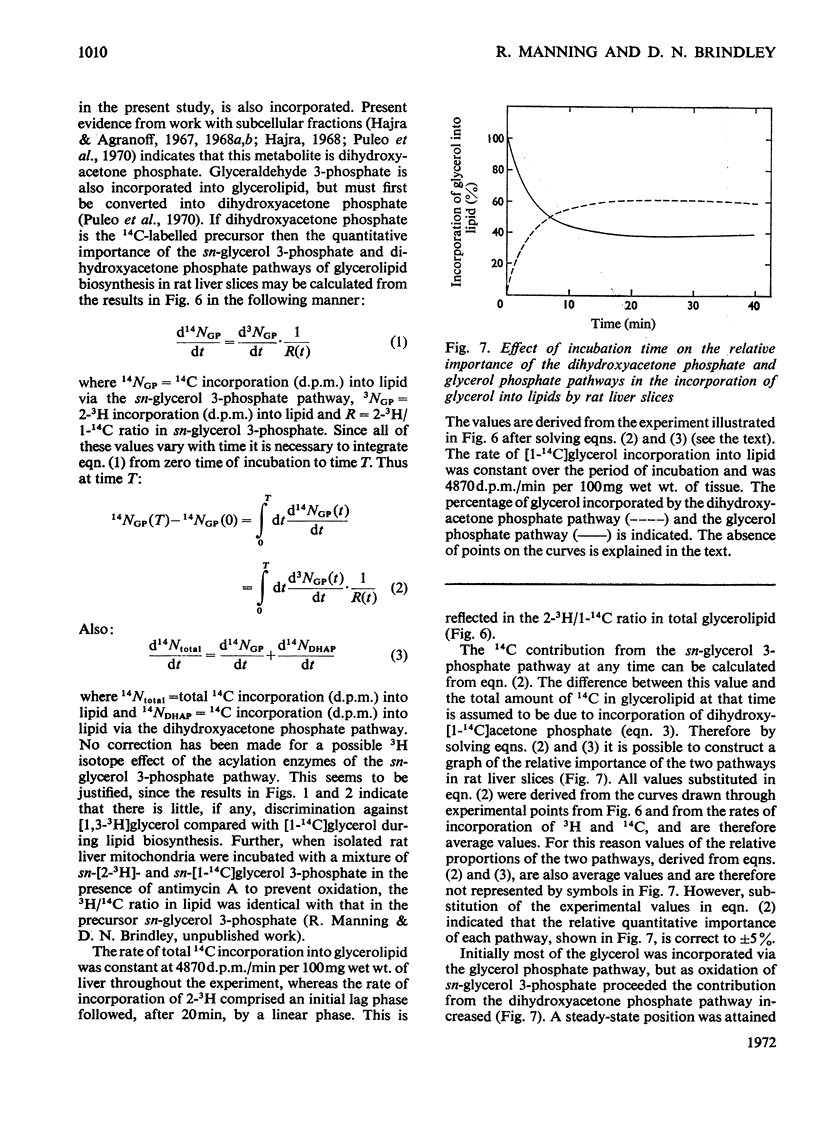
1. Rat liver slices were employed to study the relative rates of incorporation of a mixture of [2-3H]- or [1,3-3H]-glycerol and [1-14C]glycerol into lipids. 2. With 0.1mm-glycerol approx. 82% of the newly synthesized lipid, calculated from 14C incorporation, was present as neutral lipid, 13% as phosphatidylcholine and 5% as phosphatidylethanolamine. Increasing the glycerol concentration to 40mm caused a decrease in the percentage of neutral lipid to 59% and a corresponding increase in the percentage of phosphatidylcholine to 36% of the newly synthesized lipid. 3. The (d.p.m. of 2-3H)/(d.p.m. of 1-14C) ratio in glycerolipid was considerably higher than that in precursor glycerol throughout the range of experimental conditions. In contrast the incorporation of a mixture of [1,3-3H]glycerol and [1-14C]glycerol into lipid occurred with little or no change in the 3H/14C ratio. 4. Respiring rat liver mitochondria were found to oxidize a mixture of sn-[2-3H]- and sn-[1-14C]-glycerol 3-phosphate with a resultant increase in the 3H/14C ratio of the remaining sn-glycerol 3-phosphate. This increase is due to a 3H isotope effect of the mitochondrial sn-glycerol 3-phosphate dehydrogenase (EC 1.1.99.5), which discriminates against sn-[2-3H]glycerol 3-phosphate during oxidation. 5. A method is described for the simultaneous determination of the relative contributions of the glycerol phosphate and dihydroxyacetone phosphate pathways of glycerolipid biosynthesis in rat liver slices. The method involves measurement of the (d.p.m. of 2-3H)/(d.p.m. of 1-14C) ratio in both sn-glycerol 3-phosphate and glycerolipid after incubation of rat liver slices with a mixture of [2-3H]glycerol and [1-14C]glycerol for various times. 6. By using this method it was shown that 40–50% of the glycerol incorporated into lipid by rat liver slices proceeded via the sn-glycerol 3-phosphate pathway and 50–60% was incorporated via dihydroxyacetone phosphate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Hajra A. K. The acyl dihydroxyacetone phosphate pathway for glycerolipid biosynthesis in mouse liver and Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1971 Feb;68(2):411–415. doi: 10.1073/pnas.68.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brindley D. N., Hübscher G. The intracellular distribution of the enzymes catalysing the biosynthesis of glycerides in the intestinal mucosa. Biochim Biophys Acta. 1965 Dec 2;106(3):495–509. doi: 10.1016/0005-2760(65)90066-4. [DOI] [PubMed] [Google Scholar]
- Carnicero H. H., Moore C. L., Hoberman H. D. Oxidation of glycerol 3-phosphate by the perfused rat liver. J Biol Chem. 1972 Jan 25;247(2):418–426. [PubMed] [Google Scholar]
- Hajra A. K., Agranoff B. W. Acyl dihydroxyacetone phosphate. A rapidly labeled lipid in guinea pig liver mitochondria. J Biol Chem. 1967 Mar 10;242(5):1074–1075. [PubMed] [Google Scholar]
- Hajra A. K., Agranoff B. W. Acyl dihydroxyacetone phosphate. Characterization of a 32P-labeled lipid from guinea pig liver mitochondria. J Biol Chem. 1968 Apr 10;243(7):1617–1622. [PubMed] [Google Scholar]
- Hajra A. K., Agranoff B. W. Reduction of palmitoyl dihydroxyacetone phosphate by mitochondria. J Biol Chem. 1968 Jun 25;243(12):3542–3543. [PubMed] [Google Scholar]
- Hajra A. K. Biosynthesis of acyl dihydroxyacetone phosphate in guinea pig liver mitochondria. J Biol Chem. 1968 Jun 25;243(12):3458–3465. [PubMed] [Google Scholar]
- Hajra A. K., Seguin E. B., Agranoff B. W. Rapid labeling of mitochondrial lipids by labeled orthophosphate and adenosine triphosphate. J Biol Chem. 1968 Apr 10;243(7):1609–1616. [PubMed] [Google Scholar]
- Hill E. E., Lands W. E. Formation of acyl and alkenyl glycerol derivatives in Clostridium butyricum. Biochim Biophys Acta. 1970 Feb 10;202(1):209–211. doi: 10.1016/0005-2760(70)90239-0. [DOI] [PubMed] [Google Scholar]
- KENNEDY E. P. Metabolism of lipides. Annu Rev Biochem. 1957;26:119–148. doi: 10.1146/annurev.bi.26.070157.001003. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic esterification of alpha-glycerophosphate by long chain fatty acids. J Biol Chem. 1953 Sep;204(1):345–357. [PubMed] [Google Scholar]
- Liberti J. P., Jezyk P. F. Lipid biosynthesis in rat-liver slices: effects of ions, ATP and substrate concentration on glycerol incorporation. Biochim Biophys Acta. 1970 Jul 14;210(2):221–229. doi: 10.1016/0005-2760(70)90166-9. [DOI] [PubMed] [Google Scholar]
- Plackett P., Rodwell A. W. Glycerolipid biosynthesis by Mycoplasms strain Y. Biochim Biophys Acta. 1970 Jul 14;210(2):230–240. doi: 10.1016/0005-2760(70)90167-0. [DOI] [PubMed] [Google Scholar]
- Puleo L. E., Rao G. A., Reiser R. Triose phosphates as precursors of glyceride biosynthesis by rat liver microsomes. Lipids. 1970 Sep;5(9):770–775. doi: 10.1007/BF02531391. [DOI] [PubMed] [Google Scholar]
- Rao G. A., Sorrels M. F., Reiser R. Biosynthesis of triglycerides from triose phosphates by microsomes of intestinal mucosa. Lipids. 1970 Sep;5(9):762–764. doi: 10.1007/BF02531389. [DOI] [PubMed] [Google Scholar]
- Rao G. A., Sorrels M. F., Reiser R. Production and preferential utilization of dihydroxyacetone phosphate for glyceride synthesis in the presence of glycerol 3-phosphate. Biochem Biophys Res Commun. 1971 Sep;44(5):1279–1284. doi: 10.1016/s0006-291x(71)80224-3. [DOI] [PubMed] [Google Scholar]
- Robinson J., Newsholme E. A. The effects of dietary conditions and glycerol concentration on glycerol uptake by rat liver and kidney-cortex slices. Biochem J. 1969 May;112(4):449–453. doi: 10.1042/bj1120449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAUNDERS D. R., DAWSON A. M. Studies on the metabolism of glycerol by the small intestine in vitro and in vivo. Biochem J. 1962 Mar;82:477–483. doi: 10.1042/bj0820477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz F. M., Johnston J. M. The synthesis of higher glycerides via the monoglyceride pathway in hamster adipose tissue. J Lipid Res. 1971 Mar;12(2):132–138. [PubMed] [Google Scholar]
- Smith M. E., Hübscher G. The biosynthesis of glycerides by mitochondria from rat liver. The requirement for a soluble protein. Biochem J. 1966 Nov;101(2):308–316. doi: 10.1042/bj1010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavrecka M., Mitchell M. P., Hübscher G. The effect of starvation on the incorporation of palmitate into glycerides and phospholipids of rat liver homogenates. Biochem J. 1969 Nov;115(2):139–145. doi: 10.1042/bj1150139. [DOI] [PMC free article] [PubMed] [Google Scholar]


