Figure 3.
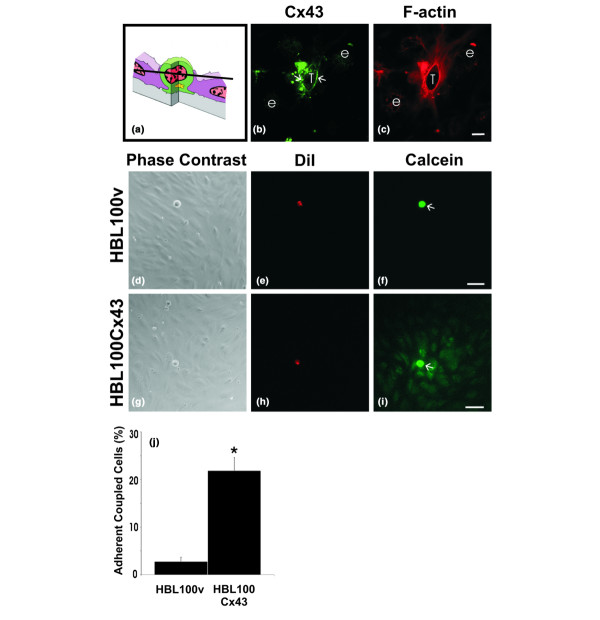
Connexin43 (Cx43) localizes to tumor/endothelial cell interfaces and assembles into functional gap junction channels. To examine Cx43 distribution during tumor cell diapedesis, cells expressing functional Cx43 (HBL100Cx43) were co-cultured with human microvascular endothelial cell (HMVEC) monolayers, fixed and labeled for (b) Cx43 (green) and (c) F-actin (red). The optical section obtained by confocal microscopy revealed (b) Cx43 (arrows) enriched at tumor cell (T)/endothelial cell (e) contact site, where it partially co-localizes with cortical F-actin. (a) Schematic diagram indicating the level of the optical section where the tumor cell is wedged between the endothelial cells and (b) the location of the Cx43-containing plaques (arrows). To evaluate if expression of exogenous Cx43 in HBL100 cells resulted in formation of functional gap junctions with HMVEC, (f) HBL100v or (i) HBL100Cx43 cells (arrows) were preloaded with (e,h) dioctadecyl-3, 3,3', 3'-tetramethylindocarbocyanine percholate (DiI; red) and (f,i) calcein-AM (green) and co-cultured with HMVEC monolayers for 30 minutes. Live observation of the co-cultures revealed that in contrast to (d-f) HBL100v cells, (g-i) HBL100Cx43 cells allowed the calcein dye to spread to adjacent HMVEC cells. (j) Gap junctional intercellular communication (GJIC) between tumor cells and endothelial cells was quantified live after 30 minutes of co-culture, revealing a 10-fold increase in the number of connexin expressing tumor cells coupled to the endothelium compared to tumor cells lacking Cx43 (*p < 0.05). Bar = (c) 10 μm and (f,i) 50 μm.
