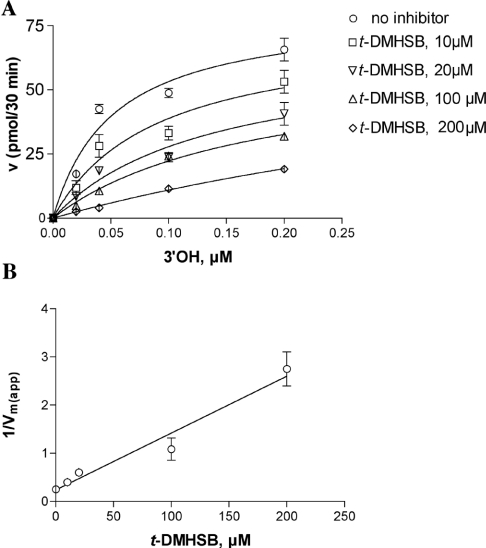
Figure 4. The resveratrol derivative t-DMHSB is a non-competitive inhibitor of pol α.
Reactions were performed under the conditions described in the Materials and methods section. (A) The initial velocities of the reaction catalysed by 0.1 unit of pol α were measured as a function of increasing poly(dA)/oligo(dT) concentrations and in the absence (○) or in the presence of 10 μM (□), 20 μM (▽), 100 μM (△) and 200 μM (◇) t-DMHSB. The DNA concentrations used (as 3′-OH ends) were 0.02, 0.04, 0.1 and 0.2 μM respectively. The TTP concentration was kept constant at 10 μM. Data were analysed as described in the Materials and methods section. Each experiment was performed in triplicate. The Ks value in the absence of inhibitor was 0.05 μM and the corresponding values in the presence of different inhibitor concentrations were 0.05 (±0.01), 0.03 (±0.01), 0.07 (±0.01) and 0.05 (±0.01) μM respectively. The Vmax(app) value in the absence of inhibitor was 3.3 (±0.1) pmol/min and the corresponding values in the presence of different inhibitor concentrations were 2.2 (±0.1), 1.6 (±0.1), 1.1 (±0.1) and 0.4 (±0.07) pmol/min respectively. (B) The variation in the apparent maximal velocity of the reaction [expressed as 1/Vmax(app)] was plotted as a function of the inhibitor concentrations. The Ki value was calculated as described in the Materials and methods section and was 20 (±2) μM. Error bars represent ±S.D.