Abstract
We have established a novel assay method based on circular dichroism that can be used for the kinetic study of the activity of amino acid racemases, such as ALR (alanine racemase). Although an enzyme-coupled assay method has been used to measure racemase activity, the CD method is superior to the enzyme assay because it can accurately determine the immediate changes of an enantiomer on racemization between its L- and D-forms. The enzyme-coupled assay requires D-amino acid oxidase, which is inactivated by an inhibitor of ALR, D-cycloserine. This indicates that the inhibitory kinetic study for ALR with D-cycloserine by the enzyme-coupled assay method is restricted to the analysis of only the reaction resulting in the formation of L-Ala from D-Ala. However, since the CD assay does not require the coupled enzyme, it can be used to comprehensively evaluate the reactions that result in the formation both of D-Ala from L-Ala and of L-Ala from D-Ala at several substrate concentrations. Streptomyces ALR also catalyses the formation of D-Ser from L-Ser and of L-Ser from D-Ser, but the catalytic constants (kcat) are 4- and 10-fold lower than those for the formation of D-Ala from L-Ala and of L-Ala from D-Ala respectively.
Keywords: alanine racemase, D-amino acid, circular dichroism, Streptomyces
Abbreviations: ALR, alanine racemase; D-CS, D-cycloserine
INTRODUCTION
D-Amino acids are found as components of the peptidoglycan layer in bacterial cell walls. They are also constituents of microbial secondary metabolites, such as an immunosuppressive agent, cyclosporin A, and an antibiotic, gramicidin S [1–3]. D-Amino acids play important roles [4–8], for example, as osmolytes in the crayfish [6] and as an energy resource in shellfish [7]. The D-forms of amino acids have also been found from mammalian sources [9–12]. In human plasma, free D-serine, D-alanine and D-proline have been identified [13]. Interestingly, increases in the amounts of D-amino acids in proteins have been observed to be associated with aging and disease, particularly with cataracts [14,15] and Alzheimer's disease [16,17] in the eyes and brain respectively. Thus, although D-amino acids are rarely present in natural resources, they certainly play significant roles physiologically.
Since the L-isomers of amino acids are generally produced in biosynthetic pathways, the formation of D-isomers can arise from equilibration of the configuration at the α-carbon starting from the L-enantiomer. ALR (alanine racemase; EC 5.1.1.1), which catalyses the racemization of both alanine enantiomers, is an essential enzyme for the synthesis of peptidoglycan, and requires pyridoxal 5′-phosphate as a cofactor [18].
D-CS (D-cycloserine; 4-amino-3-isoxazolidinone), which is produced by Streptomyces garyphalus and S. lavendulae, has been reported to competitively inhibit the catalytic activity of ALR [19,20]. On the other hand, it has been suggested by some research groups that D-CS inhibits non-competitively or causes time-dependent, irreversible inactivation of ALR [19,21]. We attempted to determine whether or not D-CS inhibits the catalytic activity of ALR in D-CS-producing micro-organisms in a recent study [22].
The enzyme-coupled assay has been conveniently used to measure the catalytic activity of some racemases [1,4,19,23–28]; however, the measured increase or decrease in the amount of NADH did not appear to be perfectly consistent with the amount of alanine racemized in both the D- to L- and the L- to D-directions when the ALR activity from D-CS-producing S. lavendulae was measured [22]. It is significant that D-CS inhibits the catalytic activity of D-amino acid oxidase, which is contained in the reaction mixture for the enzyme-coupled assay [29]. These observations made it possible to develop another method to measure ALR activity. In the present study, we establish a novel assay method for measuring the activity of racemases, including ALR, based on circular dichroism.
EXPERIMENTAL
Materials
D-Ala was purchased from Sigma Chemical Co. L-Ala, L-Ser, D-Ser, dibasic ammonium phosphate and phosphoric acid were purchased from Wako Pure Chemical Industries (Osaka, Japan). Pyridoxal 5′-phosphate, which is the cofactor of ALR, was purchased from ICN Biomedicals, Inc..
ALRs from S. lavendulae A.T.C.C. 25233 and Escherichia coli K-12 W3110 were expressed in E. coli BL21(DE3) pLysS and purified as described previously [22].
Enzyme-coupled assay to measure the catalytic activity of ALR
An enzyme-coupled assay for the measurement of ALR activity was performed according to the method described previously [25], except for the pH of the buffer and the temperature of the reaction. Production of L-Ala from D-Ala was determined in a reaction mixture (1 ml) containing 100 mM Tricine/NaOH (pH 9.1), 0.1–2.0 mM D-Ala, 0.15 unit of L-alanine dehydrogenase, 10 mM NAD+ and purified ALR (345 and 1070 ng/ml for ALRs from S. lavendulae and E. coli respectively) at 37 °C. The increase in absorbance in the reaction mixture was monitored at 340 nm (nine data points). On the other hand, the formation of D-Ala from L-Ala was followed in a reaction mixture (1 ml) containing 100 mM Tricine/NaOH (pH 9.1), 0.25–2.0 mM L-Ala, 0.12 mM NADH, 110 units of lactate dehydrogenase, 1 unit of D-amino acid oxidase and purified ALR (34.5 and 170 ng/ml for enzymes from S. lavendulae and E. coli respectively) at 37 °C. The decrease in absorbance at 340 nm was monitored.
Assay of ALR activity based on the CD spectrum
Since 100 mM Tricine/NaOH (pH 9.1), which is included in the reaction mixture for the enzyme-coupled assay, has a high absorbance in the CD assay, the solution containing ALR was dialysed against a 30 mM ammonium phosphate buffer (pH 8.2) containing pyridoxal 5′-phosphate at the same molarity as the enzyme, prior to the measurement of the CD spectrum. The solution (2 ml), which contained L-Ala and/or D-Ala at the given concentrations (0–2 mM) in a 30 mM ammonium phosphate buffer (pH 8.2), was kept at 4 °C for 1 h. To start the reaction, the enzyme (170 and 225 ng/ml for ALRs from S. lavendulae and E. coli respectively) was added to the mixture, followed by incubation at 37 °C for 10 min. The enzyme reaction was stopped by boiling for 5 min. Then 1 ml of 30 mM ammonium phosphate was added to the boiled solution, which was analysed for the CD spectrum derived from Ala. The same reaction mixture, boiled and without the 10 min incubation for the racemase reaction, was used as a control.
Measurement of CD spectra was carried out in a 1 cm-pathlength cylindrical cell at 25 °C on a JASCO JU-720 spectropolarimeter (Japan). The scan rate and bandwidth were set to 50 nm/min and 1.0 nm respectively. Spectra were averaged from eight scans and recorded between 200 and 220 nm by 0.1 nm carving. The machine sensitivity was set to 20–50 mdegrees, and the resulting spectra were treated without smoothing.
The CD assay exhibited a high reliability over a wide concentration range of the substrate, but the substrate concentration is recommended to be as low as possible for accurate measurements.
RESULTS AND DISCUSSION
Kinetic analysis using the enzyme-coupled assay
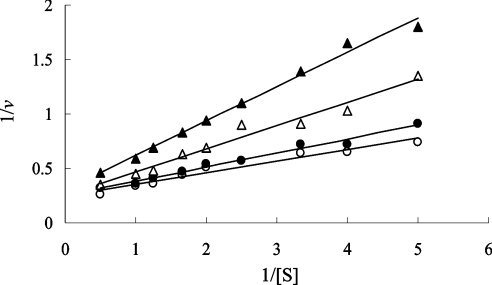
The enzyme-coupled assay has been conveniently used to measure the catalytic activity of several racemases [1,4,19,23–28]. In the present study, we attempted to estimate the kinetic parameters for ALR from D-CS-producing S. lavendulae using this assay method. Figure 1 shows double-reciprocal plots of the Streptomyces ALR reaction examined using the enzyme-coupled assay, which monitors the production of L-Ala from D-Ala in the presence of D-CS at various concentrations (0–3 mM). However, for the direction of the reaction from D-Ala to L-Ala, this enzyme-coupled assay was not able to be applied, since the D-amino acid oxidase contained in the coupled-assay mixture for the formation of D-Ala from L-Ala is inhibited by D-CS [29]. In addition, the equilibrium constant value (Keq), which was calculated from a Haldane equation defined by (kcat/Km for D-Ala)/(kcat/Km for L-Ala) [30] for ALR from S. lavendulae, was too different from 1.0 as a theoretical value. The unusual Keq values may be caused by the incompatibility of the amount of NADH between the theoretical value calculated by the variation of absorbance and the experimental value varied with the racemization of Ala in the enzyme-coupled reaction. That is, the increase or decrease in the amount of NADH may not be consistent with the amount of Ala racemized in both the D- to L- and the L- to D-directions. These considerations led us to develop a new method to measure ALR activity.
Figure 1. Lineweaver–Burk plots for the reaction of Streptomyces ALR examined using the enzyme-coupled assay, which measures L-Ala produced from D-Ala.
D-CS was added to the reaction mixture (○, 0 mM; ●, 0.5 mM; △, 1.5 mM; ▲, 3.0 mM).
Assay of racemase activity based on CD spectra
Assay methods based on the CD spectra of substrate or product have been used to determine the enzyme activity of various enzymes, e.g. triosephosphate isomerase [31], mandelate racemase [32] and ornithine decarboxylase [33]. In these cases, the kinetic parameters were calculated from only the maximum value of the CD spectrum. However, in our CD method for the ALR reaction, quantitative measurement is carried out by integrating the CD spectrum between 205 and 215 nm. Furthermore, we can obtain more reliable kinetic parameters by taking account of the reversible reaction between the substrate and product.
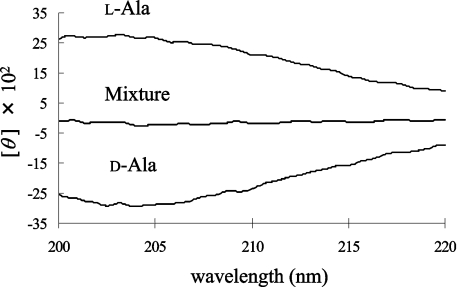
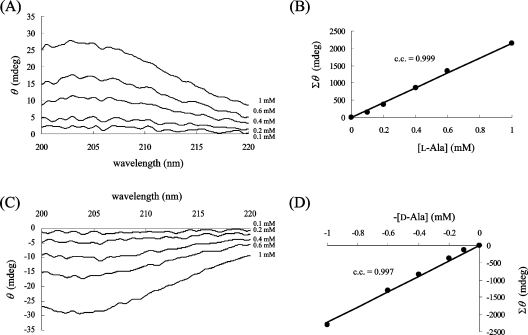
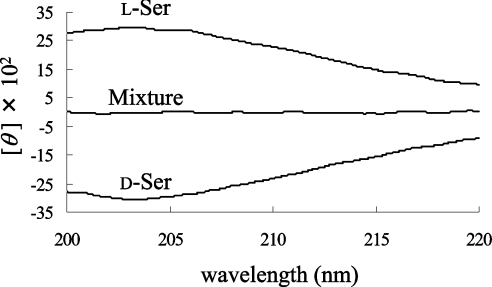
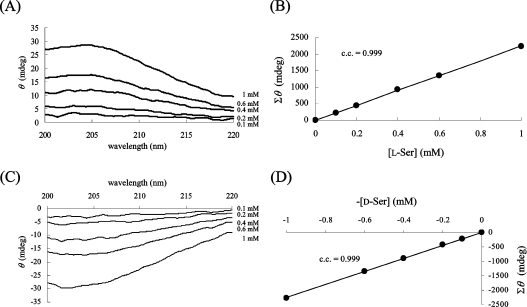
We compared the CD spectrum of D-Ala (1 mM) with that of L-Ala (1 mM). The CD spectra of D-Ala and L-Ala display symmetrical profiles against the x-axis (wavelength); however, a mixture of the enantiomers did not give a specific CD signal (Figure 2). When the CD spectrum of 1 mM L-Ala was monitored before and after the racemization reaction, the CD signal decreased as a result of the production of the isomer (results not shown). Therefore the ratio of the ALR-mediated conversion to D-Ala from L-Ala was calculated from the profile of the CD spectrum. We observed that the CD spectrum of the sample with ALR was the same as that without ALR, at least at the concentration of ALR employed in this experiment. For the quantification of Ala, each CD spectrum of the D- and L-Ala solutions at various concentrations was recorded (Figures 3A and 3C). To draw a more reliable calibration curve, all values of θ between 205 and 215 nm were integrated for every 0.1 nm for each Ala solution, and the integrated value (Σθ) was plotted against the given concentration of Ala. Since the Ala solution takes a maximum value at 203–204 nm (e.g. [θ]=2800 degrees·cm2·dmol−1 at 203.5 nm), it is preferable to integrate the surrounding areas. However, the CD spectrum between 205 and 215 nm was chosen for integration, because the signal stability is not as good at shorter wavelengths. In the resulting calibration curve, all points were closely distributed around the regression line, and a good correlation coefficient was obtained (Figures 3B and 3D). Therefore Σθ is linearly dependent on the Ala concentration, as shown in eqn (1), where P is the proportionality constant (2200 mdegrees·mM−1 in this experiment) and [D-Ala] and [L-Ala] are the D- and L-Ala concentrations (mM) respectively:
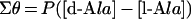 |
1 |
Figure 2. CD spectra of L-Ala, D-Ala and a mixture of the two (1 mM).
CD spectra were measured as described in the Experimental section using a 1 cm-pathlength cylindrical cell. The Ala solution gives a maximum value at 203.5 nm ([θ]=2800 degrees·cm2·dmol−1). The amino acid mixture consisting of L-Ala and D-Ala has offset signals.
Figure 3. Dependence of θ upon the concentrations of L-Ala (A, B) and D-Ala (C, D).
The CD spectra of various concentrations of L-Ala (A) and D-Ala (C) were measured. The Σθ values are precisely correlated with each Ala concentration (B, D). c.c., correlation coefficient.
Estimation of the kinetic parameters was performed as follows. Each Σθ value was calculated from the CD spectra recorded for each reaction sample, and ΔΣθ was calculated by comparing the Σθ values before and after a 10-min reaction. From the obtained △Σθ, Δ[D-Ala] (=−Δ[L-Ala]) was calculated based on eqn (1). Δ[D-Ala] per min was expressed as the reaction rate, v (mM/min), which is generally defined as shown in eqn (2) for a 10 min racemization reaction:
 |
2 |
On the other hand, the racemization reaction can be represented as follows:
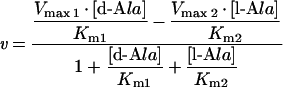 |
3 |
where Vmax1 and Km1 are the kinetic parameters for the D- to L-direction, and Vmax2 and Km2 are those for the L- to D-direction. To determine these kinetic parameters, the assay was performed with 60 or more kinds of samples containing both D- and L-Ala at various concentrations (0–2 mM). In this case, to obtain more reliable parameters, it is necessary to use as many samples as possible. The concentrations midway through the reaction were chosen as the values for [D-Ala] and [L-Ala] in eqn (3), and the v values were estimated based on ΔΣθ. The kinetic parameters were computed by numerical analysis using the least-squares method. The resultant parameters are listed in Table 1. The Keq values calculated from these data were 1.15 and 1.19 for ALRs from S. lavendulae and E. coli respectively, close to the theoretical value of 1.0.
Table 1. Kinetic parameters for alanine racemization determined by the CD assay method.
| D→L direction | L→D direction | |||
|---|---|---|---|---|
| Enzyme source | Km (mM) | kcat (min−1) | Km (mM) | kcat (min−1) |
| S. lavendulae | 0.4±0.2 | (3.8±0.4)×103 | 0.4±0.1 | (3.3±0.2)×103 |
| E. coli | 0.25±0.07 | (1.7±0.1)×103 | 0.29±0.07 | (1.66±0.03)×103 |
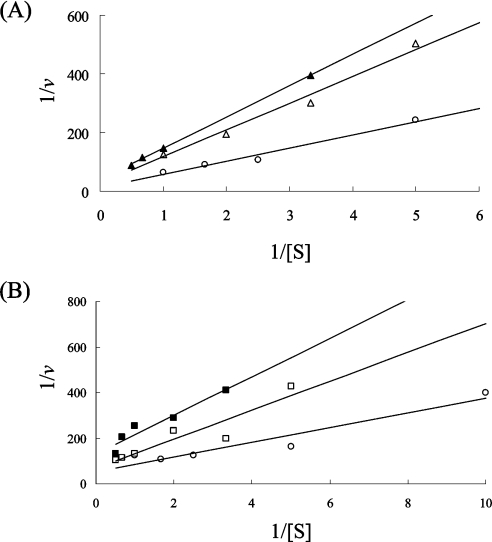
Figure 4 shows the double-reciprocal plots of the ALR reaction from D-CS-producing S. lavendulae in the presence of D-CS using the CD assay. In this Figure, the plots are based on the catalytic activity of ALR, which was measured in the reaction mixture containing one of the Ala enantiomers to compare with the result using the enzyme-coupled assay. Whereas the enzyme-coupled assay for the measurement of ALR activity is restricted to the reaction involving the formation of L-Ala from D-Ala under the condition of added D-CS, the CD assay reflects the reaction resulting in the formation both of D-Ala from L-Ala (Figure 4A) and of L-Ala from D-Ala (Figure 4B) even in the presence of the antibiotic. Thus the CD assay reflects the real enzyme reaction of ALR.
Figure 4. Lineweaver–Burk plots for the reaction of ALR from D-CS-producing S. lavendulae examined using the CD assay.
Plots for the reactions resulting in the formation of D-Ala from L-Ala (A) and of L-Ala from D-Ala (B) are shown for several substrate concentrations. D-CS was added to the reaction mixture (○), 0 mM; △, 0.05 mM; ▲, 0.1 mM; □, 0.2 mM; ■, 0.5 mM).
Advantages of the CD assay method
The new assay method established in the present study for the measurement of the catalytic activity of ALR is based on the CD spectra of both enantiomers of Ala. The method is highly quantitative and provides visible data that reflect the exhaustive reaction of ALR. We conclude that the CD assay method for the measurement of ALR activity is superior to the enzyme-coupled assay. The assay system is simple, because it does not need a coupled enzyme, such as D-amino acid oxidase contained in the enzyme-coupled assay system which is inhibited by D-CS. In the enzyme-coupled assay method, although the product generated by ALR is used as a substrate for the reverse reaction, the kinetic parameter is calculated by ignoring the reverse reaction. In the CD assay, however, we can obtain kinetic parameters without ignoring the reverse reaction.
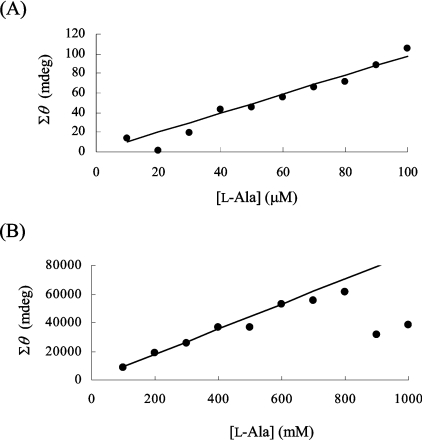
To investigate the limiting concentrations of substrate that can be used in the CD assay, CD spectra were obtained using a range of Ala concentrations, and quantification was performed as described above. The calibration curves displayed good correlation coefficients at Ala concentrations ranging from 50 μM to 400 mM using the CD assay, indicating that this CD assay method is expected to be sufficiently useful to characterize several racemases that have particular Km values. Since at high concentrations (over 10 mM) the substrate gave rise to increments of high absorbance, a 2 mm-pathlength cell was used to obtain accurate results. Figures 5(A) and 5(B) show calibration curves at low concentrations (10 μM to 100 μM Ala) and at high concentrations (100 mM to 1000 mM Ala) respectively of the Ala solution. However, it is difficult to draw the calibration curve below 10 μM. The peak of the CD signal shifted towards a longer wavelength (213 nm) at the highest Ala concentration (1000 mM), suggesting that, if the sample contains a high concentration of Ala, it should be diluted in order to obtain an adequate correlation coefficient.
Figure 5. Calibration curves for L-Ala solutions in the concentration ranges 10 μM–100 μM (A) and 100–1000 mM (B).
The Σθ values are plotted against the indicated concentration of L-Ala.
Streptomyces ALR harbours serine racemase activity
The substrate specificity of Streptomyces ALR was investigated using the CD assay method. The CD spectrum of each amino acid solution (4 mM) except for alanine and glycine was monitored before and after the addition of the Streptomyces ALR, showing that the decreased signal was observed only with the serine solution, suggesting that the Streptomyces ALR exhibits serine racemase activity. This allowed us to determine the kinetic parameters of serine racemization catalysed by ALR.
We compared the CD spectrum of D-Ser (1 mM) with that of L-Ser (1 mM) (Figure 6). The CD spectra of D-Ser and L-Ser display symmetrical profiles against the x-axis (wavelength). However, a mixture of the two enantiomers did not display a specific CD signal (Figure 6). The CD spectra of D- and L-Ser solutions at various concentrations were also recorded (Figure 7), and it was shown that the CD spectra of D- and L-Ser gave rise to the same tendency as those of D- and L-Ala. A kinetic study of racemase activity with Ser as substrate was performed using the CD assay. Since Ser is not a true substrate of Streptomyces ALR, the catalytic activity is lower than that with Ala. Therefore the added amounts of Streptomyces ALR and substrate were increased up to 300 ng/ml and up to 100 mM respectively. In addition, the incubation time was prolonged to 2 h. The kinetic parameters obtained are listed in Table 2. Another research group has recently reported that amino acid racemase from Clostridium innocuum is homologous to ALR and is predicted to exhibit serine racemase activity [34].
Figure 6. CD spectra of L-Ser, D-Ser and a mixture of the two (1 mM).
CD spectra were measured as described in the Experimental section using a 1 cm-pathlength cylindrical cell. The Ser solution gives a maximum value at 203.5 nm ([θ]=3000 degrees·cm2·dmol−1). The amino acid mixture consisting of L-Ser and D-Ser also has offset signals.
Figure 7. Dependence of θ on the concentrations of L-Ser (A, B) and D-Ser (C, D).
The CD spectra of various concentrations of L-Ser solution (A) and D-Ser solution (C) were measured. The Σθ values are precisely correlated with each serine concentration (B, D). c.c., correlation coefficient.
Table 2. Kinetic parameters for serine racemization determined by the CD assay method.
| Direction | Km (mM) | kcat (min−1) |
|---|---|---|
| D→L | 10±1 | (0.33±0.03)×103 |
| L→D | 27±6 | (0.8±0.2)×103 |
Conclusions
The CD assay method, established in the present study, will be a powerful tool for the characterization of the enzymes that catalyse racemization between the D- and L-forms of amino acids. In addition, this CD method may be useful to characterize the activities of some amino acid racemases in diseases related to D-amino acids.
Acknowledgments
This work was supported by the National Project on Protein Structural and Functional Analyses, Japan.
References
- 1.Hoffmann K., Schneider-Scherzer E., Kleinkauf H., Zocher R. Purification and characterization of eukaryotic alanine racemase acting as key enzyme in cyclosporin biosysthesis. J. Biol. Chem. 1994;269:12710–12714. [PubMed] [Google Scholar]
- 2.Vater J., Kleinkauf H. Gramicidin S-synthetase. A further characterization of phenylalanine racemase, the light enzyme of gramicidin S-synthetase. Biochim. Biophys. Acta. 1976;429:1062–1072. doi: 10.1016/0005-2744(76)90351-x. [DOI] [PubMed] [Google Scholar]
- 3.Roskoski R., Kleinkauf H., Gevers W., Lippmann F. Tyrocidine biosynthesis by three complementary fractions from Bacillus brevis (ATCC 8185) Biochemistry. 1970;9:4839–4845. doi: 10.1021/bi00827a002. [DOI] [PubMed] [Google Scholar]
- 4.Nomura T., Yamamoto I., Morishita F., Furukawa Y., Matsushima O. Purification and some properties of alanine racemase from a bivalve mollusc Carbicula japonica. J. Exp. Zool. 2001;289:1–9. doi: 10.1002/1097-010x(20010101/31)289:1<1::aid-jez1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe T., Shibata K., Kera Y., Yamada R. Occurrence of free D-aspartate and aspartate racemase in the blood shell Scapharca broughtonii. Amino Acids. 1998;14:353–360. doi: 10.1007/BF01318854. [DOI] [PubMed] [Google Scholar]
- 6.Shibata K., Shirasuna K., Motegi K., Kera Y., Abe H., Yamada R. Purification and properties of alanine racemase from crayfish Procambarus clarkii. Comp. Biochem. Physiol. B. 2000;126:599–608. doi: 10.1016/s0305-0491(00)00228-5. [DOI] [PubMed] [Google Scholar]
- 7.Shibata K., Watanabe T., Yoshikawa H., Abe K., Takahashi S., Kera Y., Yamada R. Purification and characterization of aspartate racemase from bivalve mollusk Scapharca broughtonii. Comp. Biochem. Physiol. B. 2003;134:307–314. doi: 10.1016/s1096-4959(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa N., Dhomae N., Takio K., Abe H. Purification, properties, and partial amino acid sequences of alanine racemase from the muscle of the black tiger prawn Penaeus monodon. Comp. Biochem. Physiol. B. 2002;133:445–453. doi: 10.1016/s1096-4959(02)00187-2. [DOI] [PubMed] [Google Scholar]
- 9.Nagata Y., Akino T., Ohno K. The presence of free D-amino acids in mouse tissues. Experientia. 1989;45:330–332. doi: 10.1007/BF01957466. [DOI] [PubMed] [Google Scholar]
- 10.Nagata Y., Horiike K., Maeda T. Distribution of free D-serine in vertebrate brains. Brain Res. 1994;634:291–295. doi: 10.1016/0006-8993(94)91932-1. [DOI] [PubMed] [Google Scholar]
- 11.Fujii N. D-amino acids in living higher organisms. Origin Life Evol. Biosphere. 2002;32:103–127. doi: 10.1023/a:1016031014871. [DOI] [PubMed] [Google Scholar]
- 12.Wang L. Z., Zhu X. Z. Spatiotemporal relationships among D-serine, serine racemase, and D-amino acid oxidase during mouse postnatal development. Acta Pharmacol. Sin. 2003;24:965–974. [PubMed] [Google Scholar]
- 13.Nagata Y., Masui R., Akino T. The presence of free D-serine, D-alanine and D-proline in human plasma. Experientia. 1992;48:986–988. doi: 10.1007/BF01919147. [DOI] [PubMed] [Google Scholar]
- 14.Fujii N., Momose Y., Ishii N., Takita M., Akaboshi M., Kodama M. The mechanisms of simultaneous stereoinversion, racemization, and isomerization at specific aspartyl residues of aged lens proteins. Mech. Ageing Dev. 1999;107:347–358. doi: 10.1016/s0047-6374(98)00129-8. [DOI] [PubMed] [Google Scholar]
- 15.Takemoto L., Fujii H., Boyle D. Mechanism of asparagine deamidation during human senile cataractogenesis. Exp. Eye Res. 2001;72:559–563. doi: 10.1006/exer.2001.0983. [DOI] [PubMed] [Google Scholar]
- 16.Nagata Y., Borghi M., Fisher G. H., D'Aniello A. Free D-serine concentration in normal and Alzheimer human brain. Brain Res. Bull. 1985;38:181–183. doi: 10.1016/0361-9230(95)00087-u. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto K., Fukushima T., Shimizu E., Okada S., Komatsu N., Okamura N., Koike K., Koizumi H., Kumakiri C., Imai K., Iyo M. Possible role of D-serine in the pathophysiology of Alzheimer's disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2004;28:385–388. doi: 10.1016/j.pnpbp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Walsh C. T. Enzymes in the D-alanine branch of bacterial cell wall peptidoglycan assembly. J. Biol. Chem. 1989;264:2393–2396. [PubMed] [Google Scholar]
- 19.Wang E., Walsh C. Suicide substrates for the alanine racemase of Escherichia coli B. Biochemistry. 1978;17:1313–1321. doi: 10.1021/bi00600a028. [DOI] [PubMed] [Google Scholar]
- 20.Lambert M. P., Neuhaus F. C. Mechanism of D-cycloserine action: alanine racemase from Escherichia coli W. J. Bacteriol. 1972;110:978–987. doi: 10.1128/jb.110.3.978-987.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenn T. D., Stamper G. F., Morollo A. A., Ringe D. A side reaction of alanine racemase: transamination of cycloserine. Biochemistry. 2003;42:5775–5783. doi: 10.1021/bi027022d. [DOI] [PubMed] [Google Scholar]
- 22.Noda M., Kawahara Y., Ichikawa A., Matoba Y., Matsuo H., Lee D.-G., Kumagai T., Sugiyama M. Self-protection mechanism in D-cycloserine-producing Streptomyces lavendulae: gene cloning, characterization, and kinetics of its alanine racemase and D-alanyl-D-alanine ligase, which are target enzymes of D-cycloserine. J. Biol. Chem. 2004;279:46143–46152. doi: 10.1074/jbc.M404603200. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe A., Kurokawa Y., Yoshimura T., Esaki N. Role of tyrosine 265 of alanine racemase from Bacillus stearothermophilus. J. Biochem. (Tokyo) 1999;125:987–990. doi: 10.1093/oxfordjournals.jbchem.a022406. [DOI] [PubMed] [Google Scholar]
- 24.Wassermann S. A., Daub E., Grisafi P., Botstein D., Walsh C. T. Catabolic alanine racemase from Salmonella typhimurium: DNA sequence, enzyme purification and characterization. Biochemistry. 1984;23:5182–5187. doi: 10.1021/bi00317a015. [DOI] [PubMed] [Google Scholar]
- 25.Esaki N., Walsh C. T. Biosynthetic alanine racemase of Salmonella typhimurium: purification and characterization of the enzyme encoded by the alr gene. Biochemistry. 1986;25:3261–3267. doi: 10.1021/bi00359a027. [DOI] [PubMed] [Google Scholar]
- 26.Yokoigawa K., Okubo Y., Soda K. Subunit interaction of monomeric alanine racemases from four shigella species in catalytic reaction. FEMS Microbiol. Lett. 2003;221:263–267. doi: 10.1016/S0378-1097(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 27.Inagaki K., Tanizawa K., Badet B., Walsh C. T., Tanaka H., Soda K. Thermostable alanine racemase from Bacillus stearothermophilus: molecular cloning of the gene, enzyme purification and characterization. Biochemistry. 1986;25:3268–3274. doi: 10.1021/bi00359a028. [DOI] [PubMed] [Google Scholar]
- 28.Patrick W. M., Weisner J., Blackburn J. M. Site-directed mutagenesis of Tyr354 in Geobacillus stearothermophilus alanine racemase identifies a role in controlling substrate specificity and a possible role in the evolution of antibiotic resistance. ChemBioChem. 2002;8:789–792. doi: 10.1002/1439-7633(20020802)3:8<789::AID-CBIC789>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 29.Frecking M. G., Hoeprich P. D. Effect of cycloserine on D-amino acid oxidase. Arch. Biochem. Biophys. 1966;115:108–111. doi: 10.1016/s0003-9861(66)81045-7. [DOI] [PubMed] [Google Scholar]
- 30.Briggs G. E., Haldane J. B. S. A note on the kinetics of enzyme action. Biochem. J. 1925;19:338–339. doi: 10.1042/bj0190338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahey R. C., Fischer E. F. A nonenzyme-coupled assay for triosephosphate isomerase based upon circular dichroism of glyceraldehyde-3-phosphate. Anal. Biochem. 1974;57:547–554. doi: 10.1016/0003-2697(74)90109-2. [DOI] [PubMed] [Google Scholar]
- 32.Sharp T. R., Hegeman G. D., Kenyon G. L. A direct kinetic assay for mandelate racemase using circular dichroic measurements. Anal. Biochem. 1979;94:329–334. doi: 10.1016/0003-2697(79)90368-3. [DOI] [PubMed] [Google Scholar]
- 33.Brooks H. B., Phillips M. A. Circular dichroism assay for decarboxylation of optically pure amino acids: application to ornithine decarboxylase. Anal. Biochem. 1996;238:191–194. doi: 10.1006/abio.1996.0274. [DOI] [PubMed] [Google Scholar]
- 34.David V., Bozdogan B., Mainardi J.-L., Legrand R., Gutmann L., Leclercq R. Mechanism of intrinsic resistance to vancomycin in Clostridium innocuum NCIB 10674. J. Bacteriol. 2004;186:3415–3422. doi: 10.1128/JB.186.11.3415-3422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]