Abstract
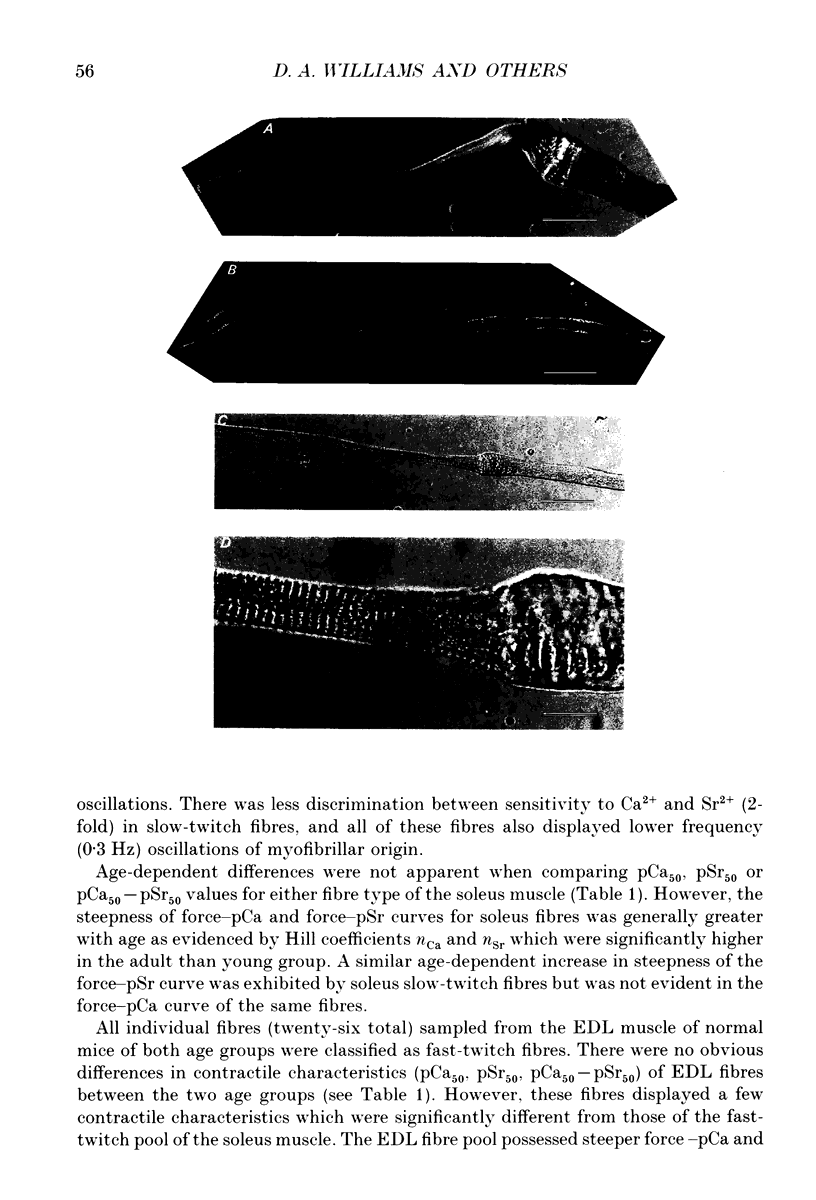
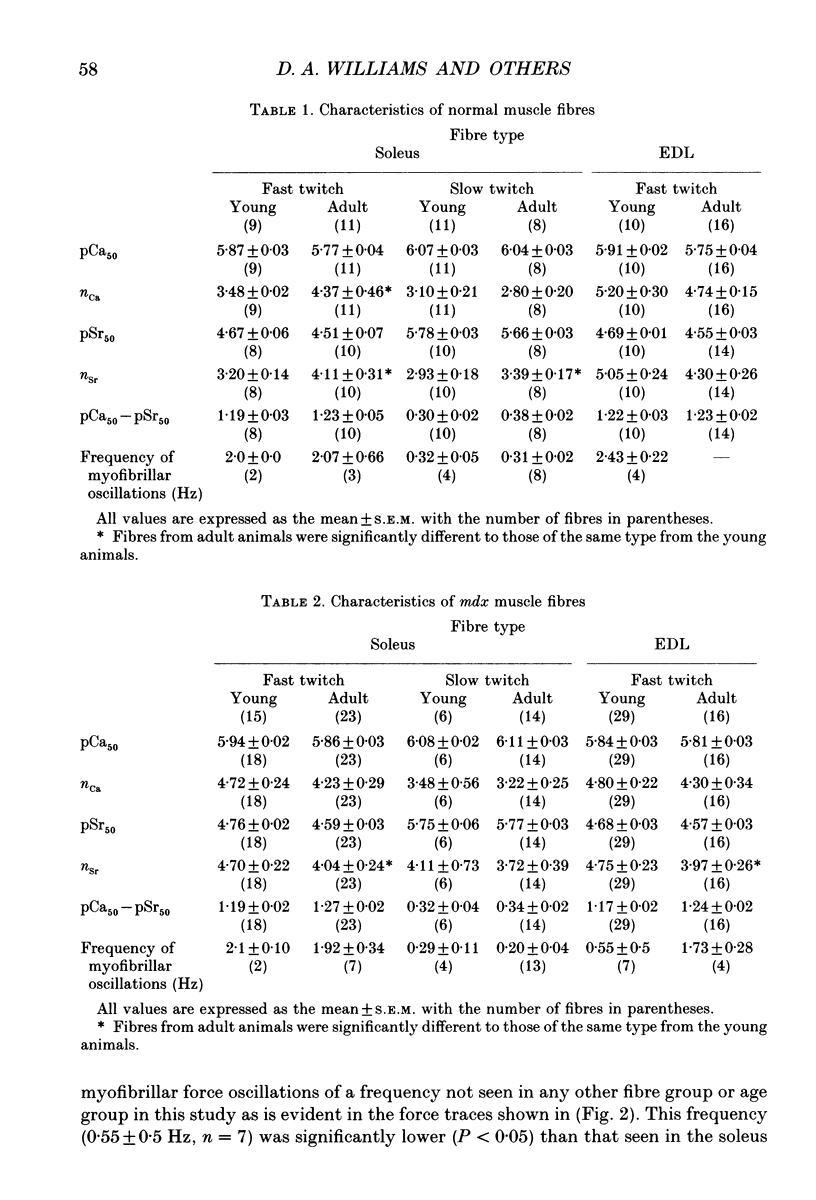
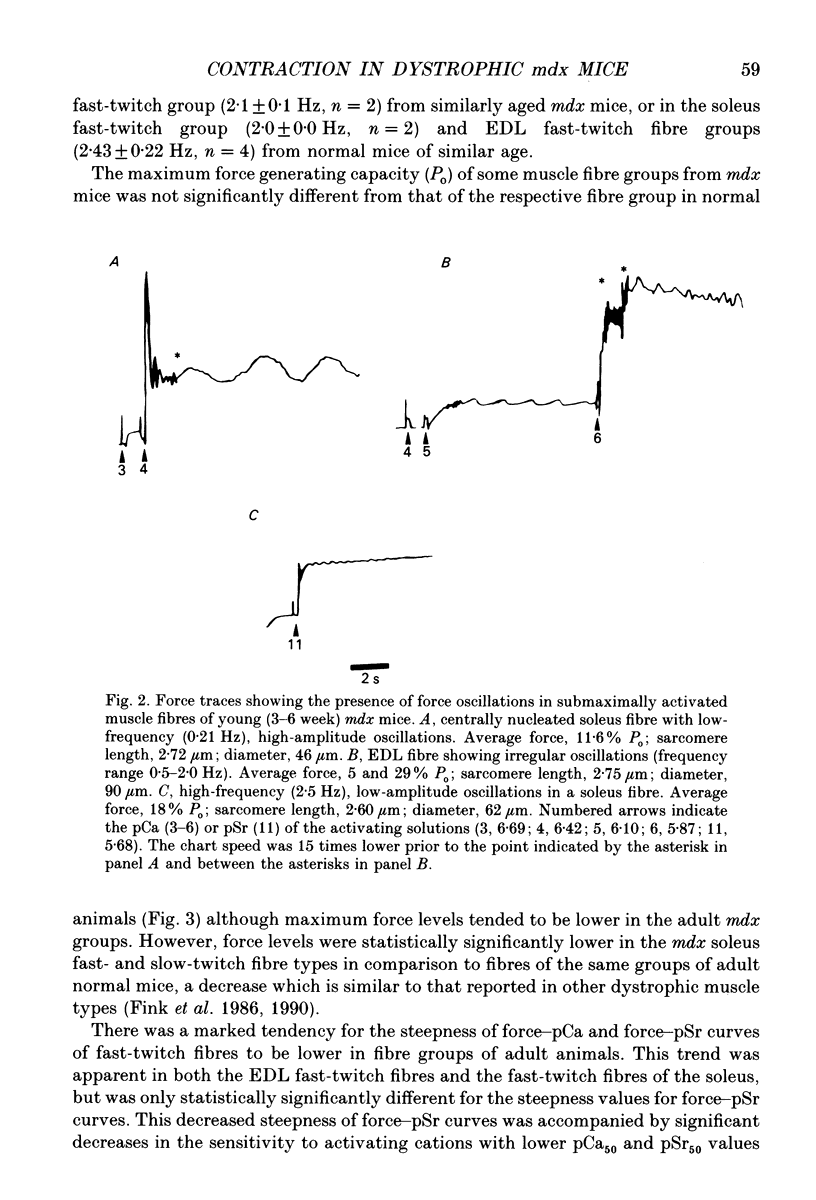
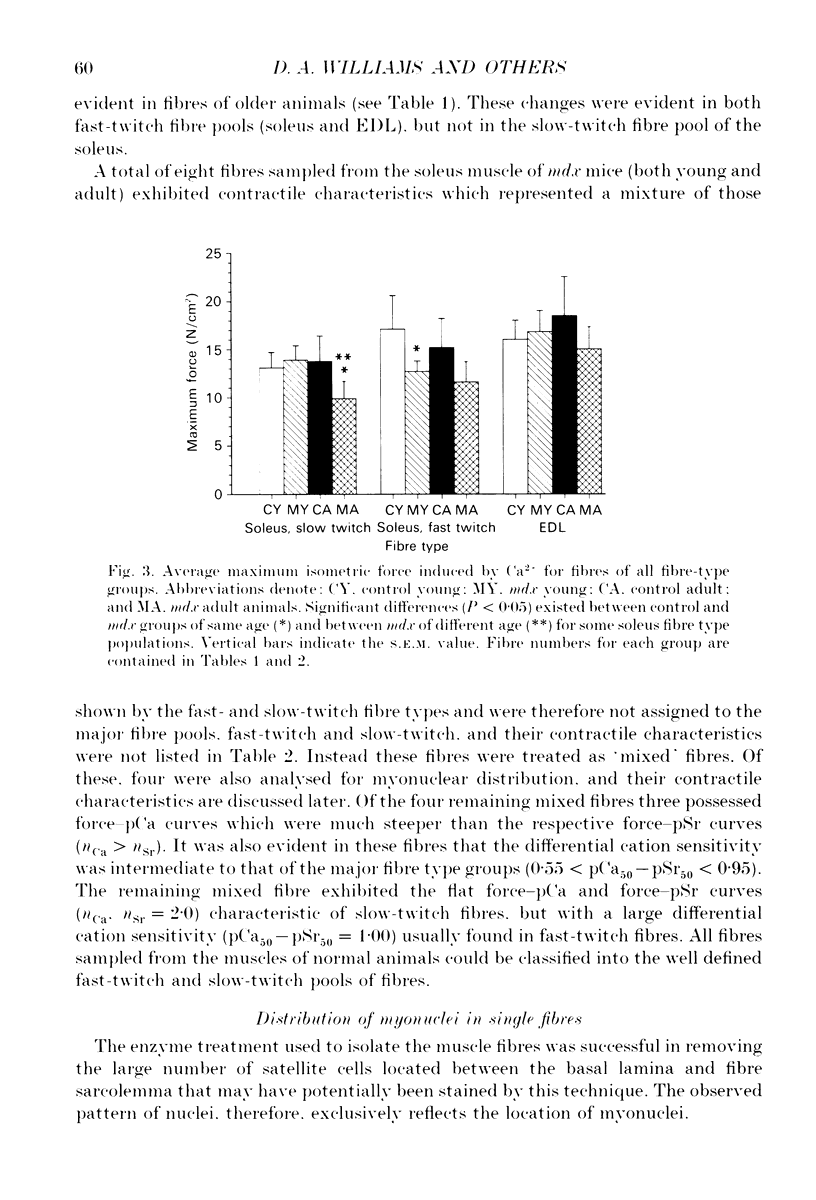
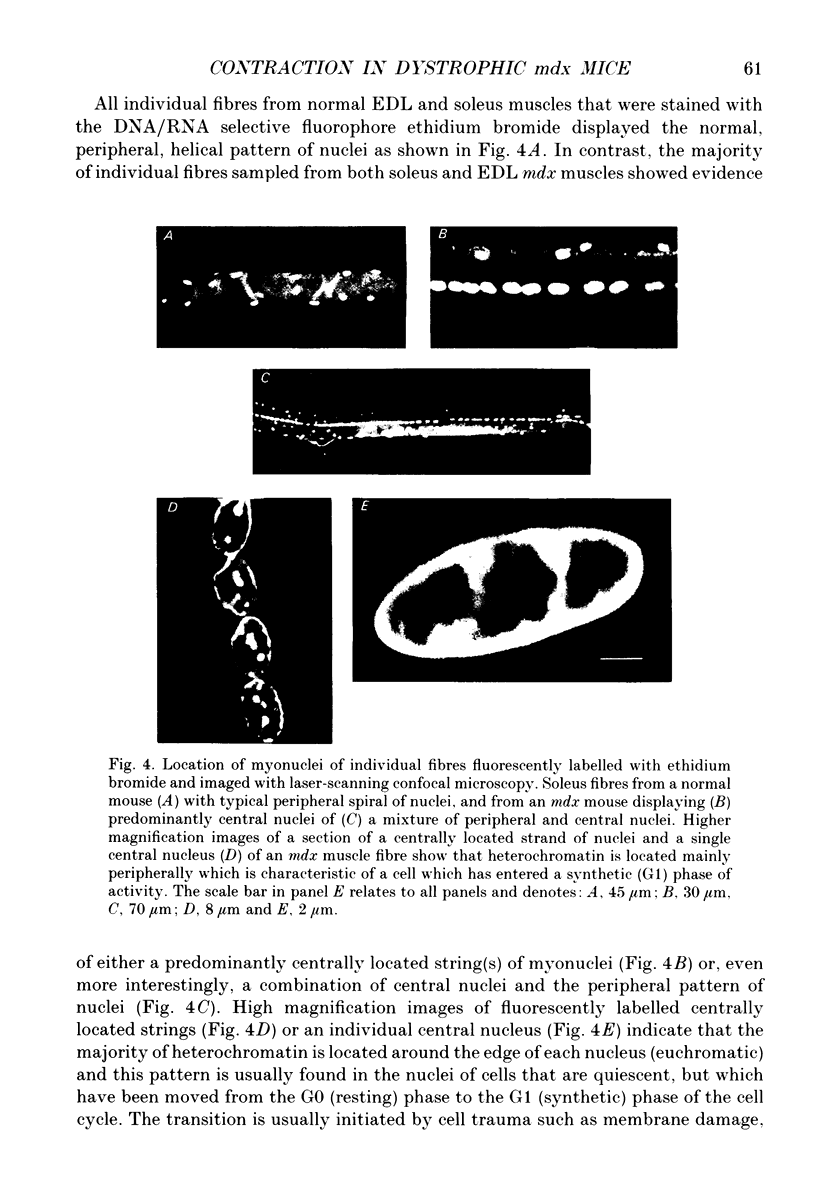
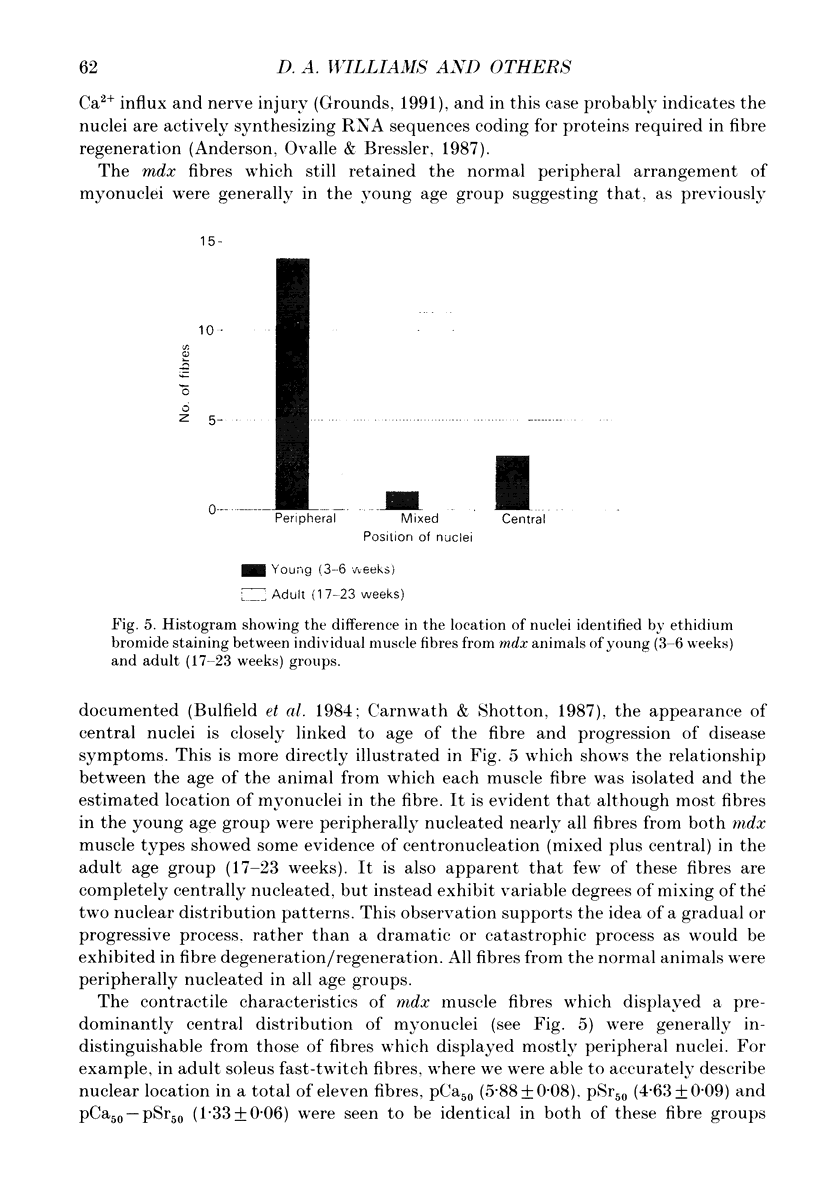
1. Single muscle fibres were enzymatically isolated from the soleus and extensor digitorum longus (EDL) muscles of genetically dystrophic mdx and normal (C57BL/10) mice aged 3-6 or 17-23 weeks. 2. Fibres of both muscles were chemically skinned with the non-ionic detergent Triton X-100 (2% v/v). Ca(2+)- and Sr(2+)-activated contractile responses were recorded and comparisons were made between several contractile parameters of various fibre types of normal and dystrophic mice of similar age. 3. There were no significant differences in the following contractile parameters of skinned fibres of normal and mdx mice of the same age: sensitivity to activating Ca2+ (pCa50) or Sr2+ (pSr50) and differential sensitivity to the activating ions (pCa50-pSr50). However the maximum isometric tension (Po) and the frequency of myofibrillar force oscillations in EDL fast-twitch fibres of young mdx mice were significantly lower than those of soleus fast-twitch fibres of the same animals, or fast-twitch fibres (EDL or soleus) of normal mice. 4. Age-related differences were apparent in some contractile parameters of both normal and mdx mice. In particular the steepness of force-pCa and force-pSr curves increased with age in normal mice, yet decreased with age in fibres of mdx mice. 5. A fluorescent probe, ethidium bromide, which interchelates with DNA, was used with laser-scanning confocal microscopy to determine the distribution of myonuclei in fibres. Fibres isolated from either muscle type of normal animals displayed a characteristic peripheral spiral of myonuclei. Fibres from muscles of mdx mice displayed three major patterns of nuclear distribution; the normal peripheral spiral, long central strands of nuclei, and a mixture of these two patterns. 6. The contractile characteristics of mdx fibres were not markedly influenced by the nuclear distribution pattern in that there were no discernible differences in the major contractile parameters (the Hill coefficients nCa and nSr, which are associated with the steepness of the Ca2+ and Sr2+ activation curves, pCa50, pSr50, pCa50-pSr50) of skinned fibres possessing peripheral or central nuclei. However, except for nSr, these values were all lower in individual fibres which displayed similar proportions of central and peripheral nuclei. The presence of mixed nucleation and absence of fibres with embryonic contractile characteristics in mdx mice suggest that the dystrophin-negative fibres can repair locally occurring muscle damage.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. E., Bressler B. H., Ovalle W. K. Functional regeneration in the hindlimb skeletal muscle of the mdx mouse. J Muscle Res Cell Motil. 1988 Dec;9(6):499–515. doi: 10.1007/BF01738755. [DOI] [PubMed] [Google Scholar]
- Anderson J. E., Ovalle W. K., Bressler B. H. Electron microscopic and autoradiographic characterization of hindlimb muscle regeneration in the mdx mouse. Anat Rec. 1987 Nov;219(3):243–257. doi: 10.1002/ar.1092190305. [DOI] [PubMed] [Google Scholar]
- Beam K. G. Duchenne muscular dystrophy. Localizing the gene product. Nature. 1988 Jun 30;333(6176):798–799. doi: 10.1038/333798a0. [DOI] [PubMed] [Google Scholar]
- Bulfield G., Siller W. G., Wight P. A., Moore K. J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnwath J. W., Shotton D. M. Muscular dystrophy in the mdx mouse: histopathology of the soleus and extensor digitorum longus muscles. J Neurol Sci. 1987 Aug;80(1):39–54. doi: 10.1016/0022-510x(87)90219-x. [DOI] [PubMed] [Google Scholar]
- Dangain J., Vrbova G. Muscle development in mdx mutant mice. Muscle Nerve. 1984 Nov-Dec;7(9):700–704. doi: 10.1002/mus.880070903. [DOI] [PubMed] [Google Scholar]
- Fink R. H., Stephenson D. G., Williams D. A. Calcium and strontium activation of single skinned muscle fibres of normal and dystrophic mice. J Physiol. 1986 Apr;373:513–525. doi: 10.1113/jphysiol.1986.sp016060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R. H., Stephenson D. G., Williams D. A. Physiological properties of skinned fibres from normal and dystrophic (Duchenne) human muscle activated by Ca2+ and Sr2+. J Physiol. 1990 Jan;420:337–353. doi: 10.1113/jphysiol.1990.sp017916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glesby M. J., Rosenmann E., Nylen E. G., Wrogemann K. Serum CK, calcium, magnesium, and oxidative phosphorylation in mdx mouse muscular dystrophy. Muscle Nerve. 1988 Aug;11(8):852–856. doi: 10.1002/mus.880110809. [DOI] [PubMed] [Google Scholar]
- Grounds M. D. Towards understanding skeletal muscle regeneration. Pathol Res Pract. 1991 Jan;187(1):1–22. doi: 10.1016/S0344-0338(11)81039-3. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Johnson M. A. Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin Exp Pharmacol Physiol. 1978 Nov-Dec;5(6):587–600. doi: 10.1111/j.1440-1681.1978.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Head S. I., Stephenson D. G., Williams D. A. Properties of enzymatically isolated skeletal fibres from mice with muscular dystrophy. J Physiol. 1990 Mar;422:351–367. doi: 10.1113/jphysiol.1990.sp017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head S. I., Williams D. A., Stephenson D. G. Abnormalities in structure and function of limb skeletal muscle fibres of dystrophic mdx mice. Proc Biol Sci. 1992 May 22;248(1322):163–169. doi: 10.1098/rspb.1992.0058. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Huard J., Labrecque C., Dansereau G., Robitaille L., Tremblay J. P. Dystrophin expression in myotubes formed by the fusion of normal and dystrophic myoblasts. Muscle Nerve. 1991 Feb;14(2):178–182. doi: 10.1002/mus.880140213. [DOI] [PubMed] [Google Scholar]
- Jacoby J., Ko K., Weiss C., Rushbrook J. I. Systematic variation in myosin expression along extraocular muscle fibres of the adult rat. J Muscle Res Cell Motil. 1990 Feb;11(1):25–40. doi: 10.1007/BF01833323. [DOI] [PubMed] [Google Scholar]
- Law P. K., Goodwin T. G., Wang M. G. Normal myoblast injections provide genetic treatment for murine dystrophy. Muscle Nerve. 1988 Jun;11(6):525–533. doi: 10.1002/mus.880110602. [DOI] [PubMed] [Google Scholar]
- Love D. R., Morris G. E., Ellis J. M., Fairbrother U., Marsden R. F., Bloomfield J. F., Edwards Y. H., Slater C. P., Parry D. J., Davies K. E. Tissue distribution of the dystrophin-related gene product and expression in the mdx and dy mouse. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3243–3247. doi: 10.1073/pnas.88.8.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G. S., Stephenson D. G., Williams D. A. Endurance exercise effects on the contractile properties of single, skinned skeletal muscle fibres of young rats. Pflugers Arch. 1991 Mar;418(1-2):161–167. doi: 10.1007/BF00370466. [DOI] [PubMed] [Google Scholar]
- Marshall P. A., Williams P. E., Goldspink G. Accumulation of collagen and altered fiber-type ratios as indicators of abnormal muscle gene expression in the mdx dystrophic mouse. Muscle Nerve. 1989 Jul;12(7):528–537. doi: 10.1002/mus.880120703. [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989 Jan 12;337(6203):176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Cody S. H., Gehring C. A., Parish R. W., Harris P. J. Confocal imaging of ionised calcium in living plant cells. Cell Calcium. 1990 Apr;11(4):291–297. doi: 10.1016/0143-4160(90)90006-g. [DOI] [PubMed] [Google Scholar]
- Williams D. A. Quantitative intracellular calcium imaging with laser-scanning confocal microscopy. Cell Calcium. 1990 Oct;11(9):589–597. doi: 10.1016/0143-4160(90)90013-k. [DOI] [PubMed] [Google Scholar]