Abstract
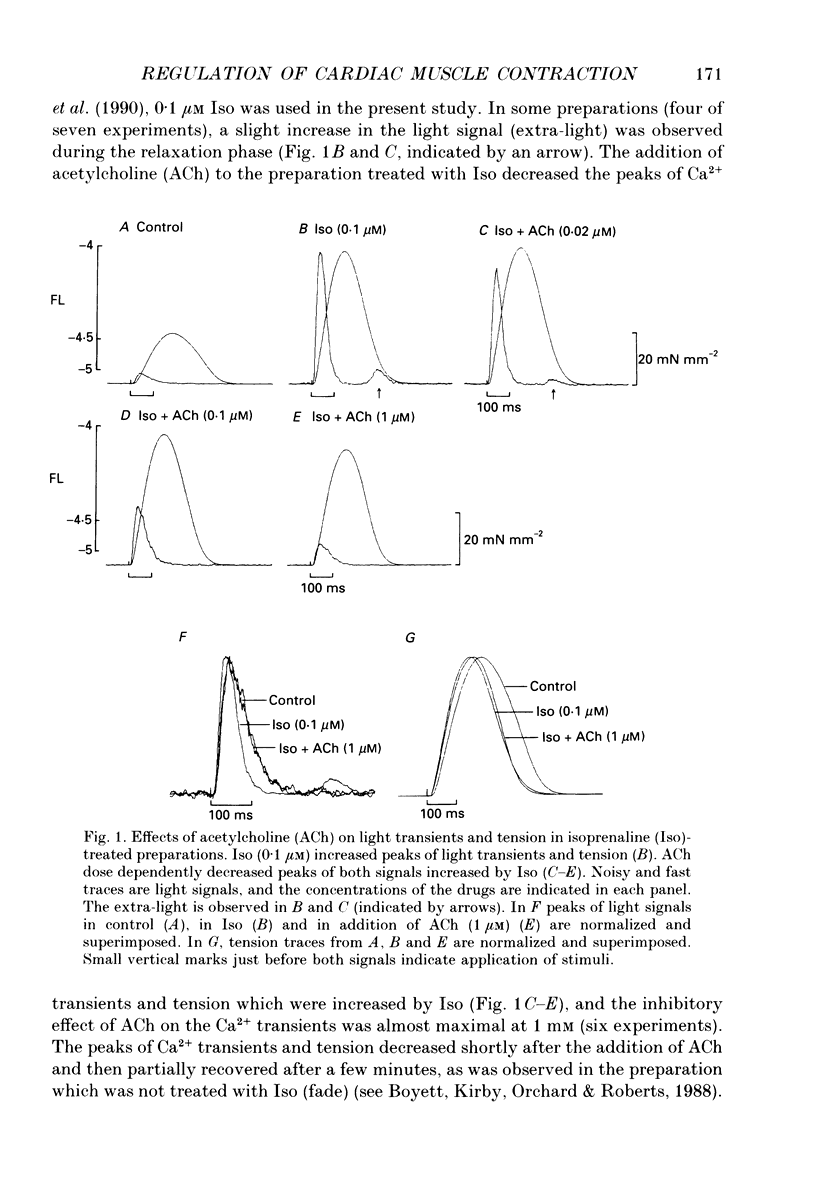
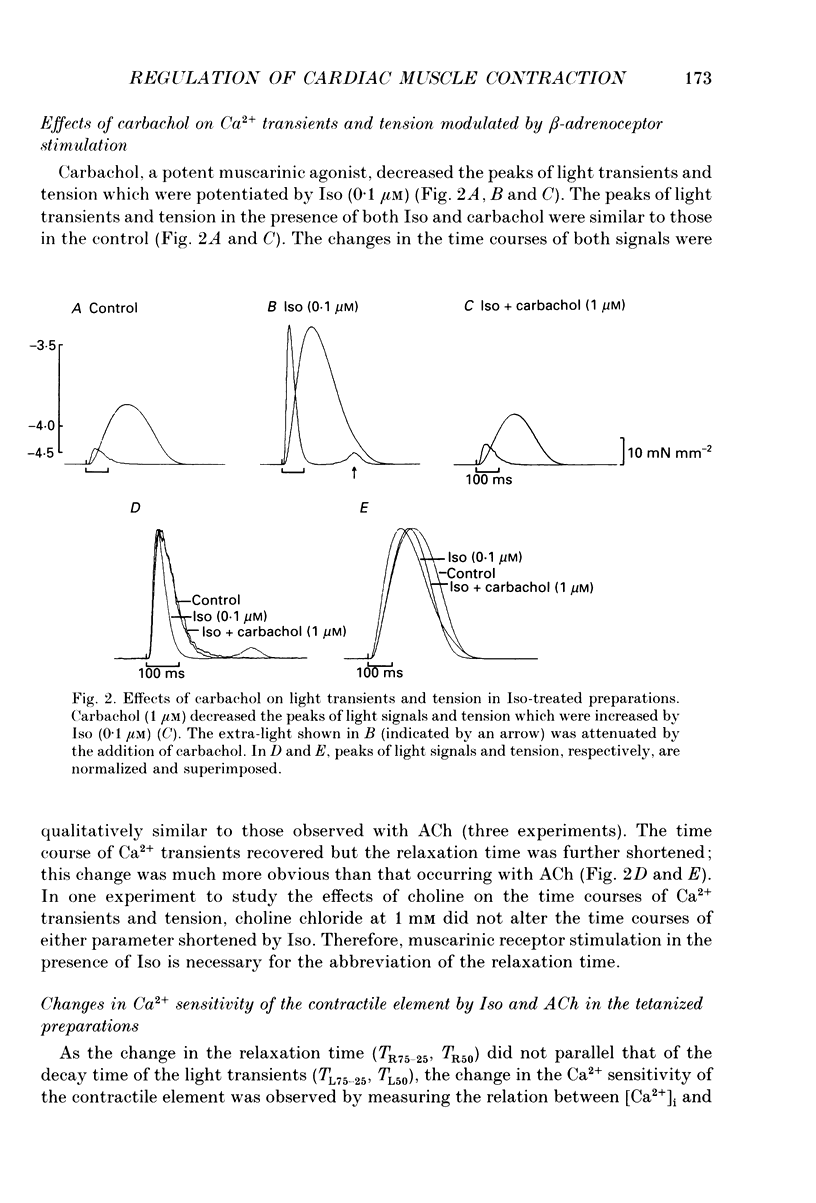
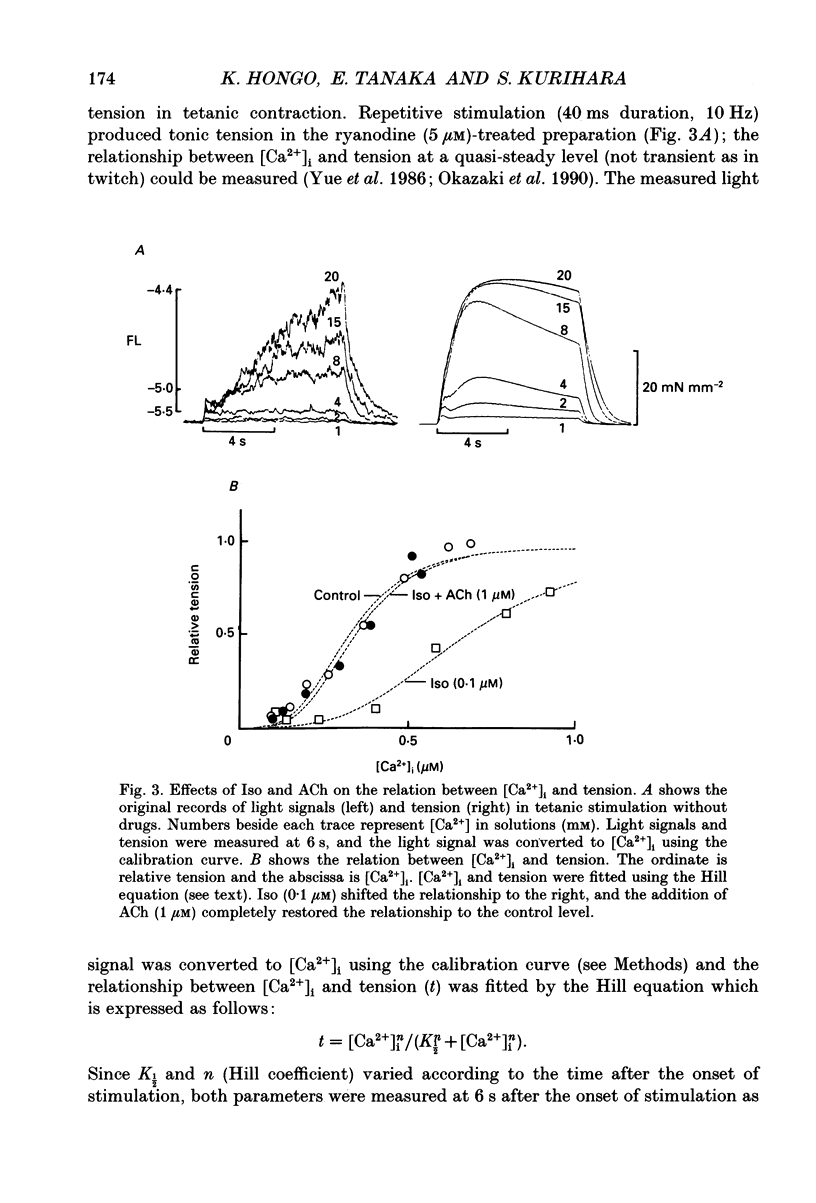
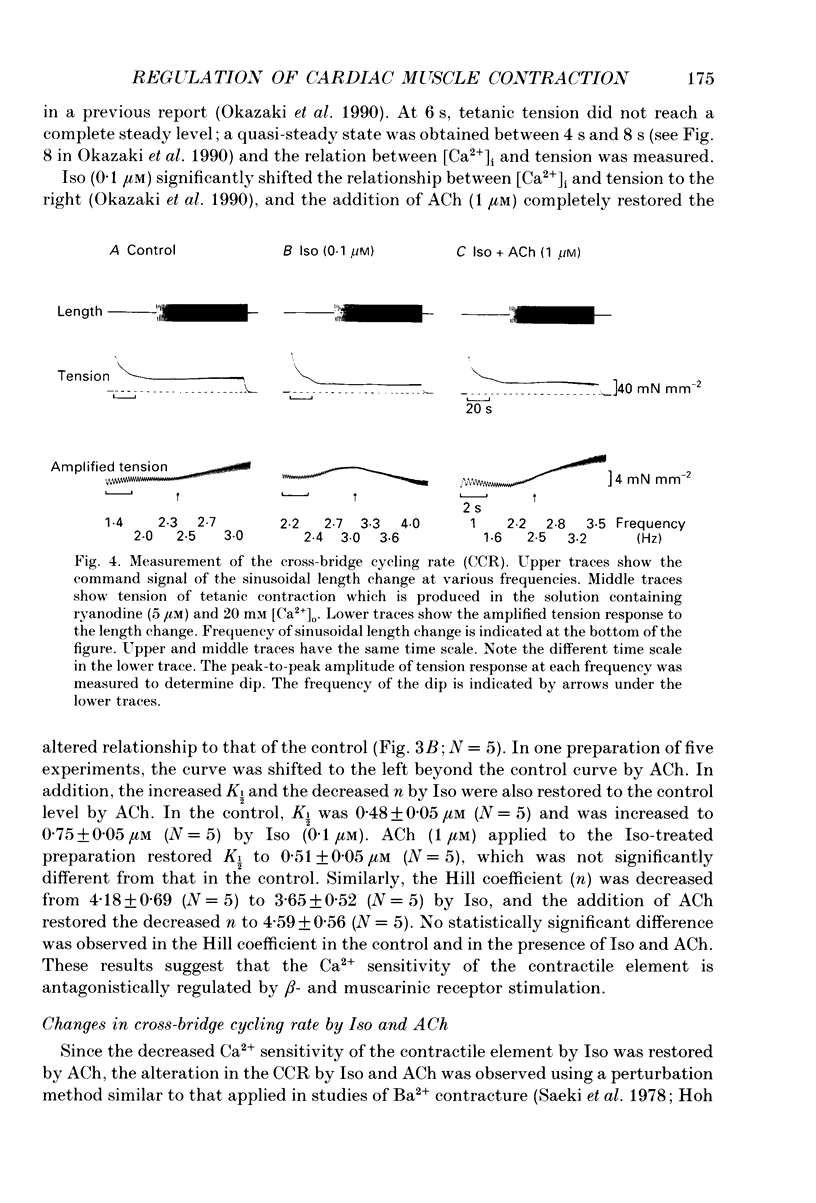
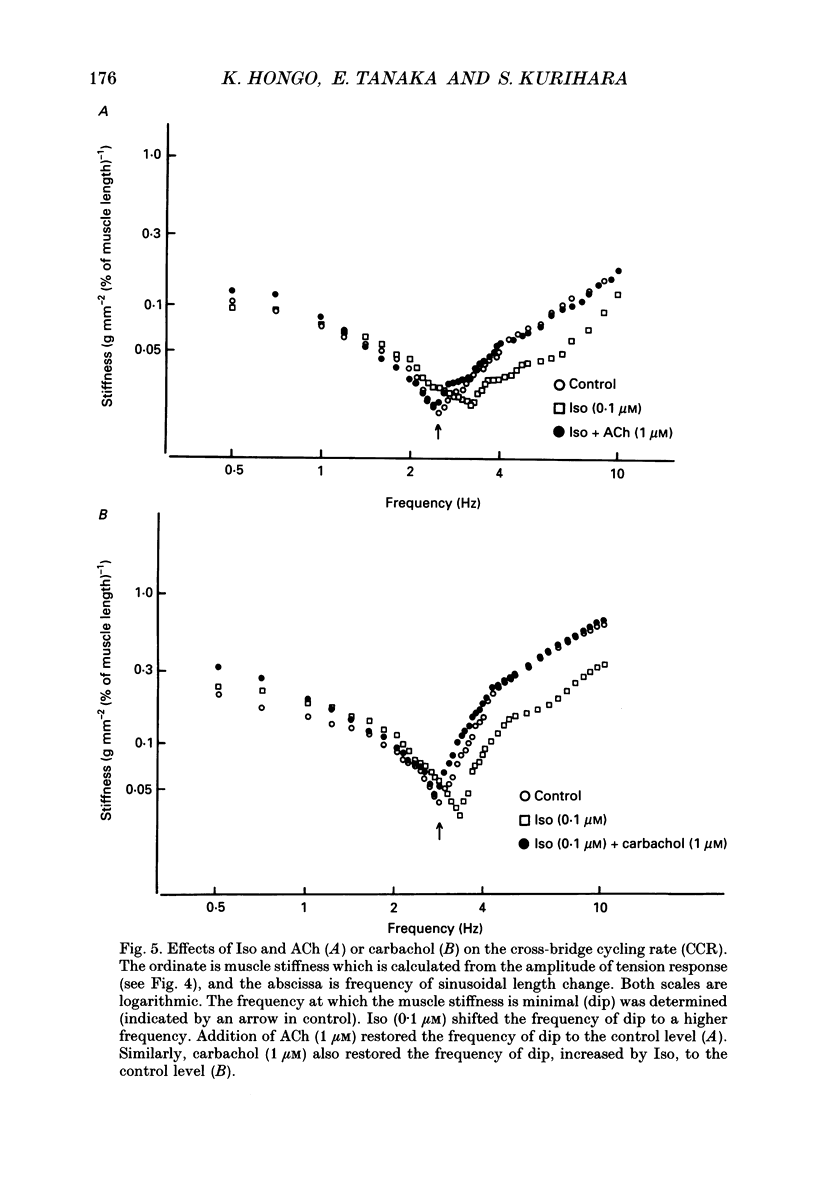
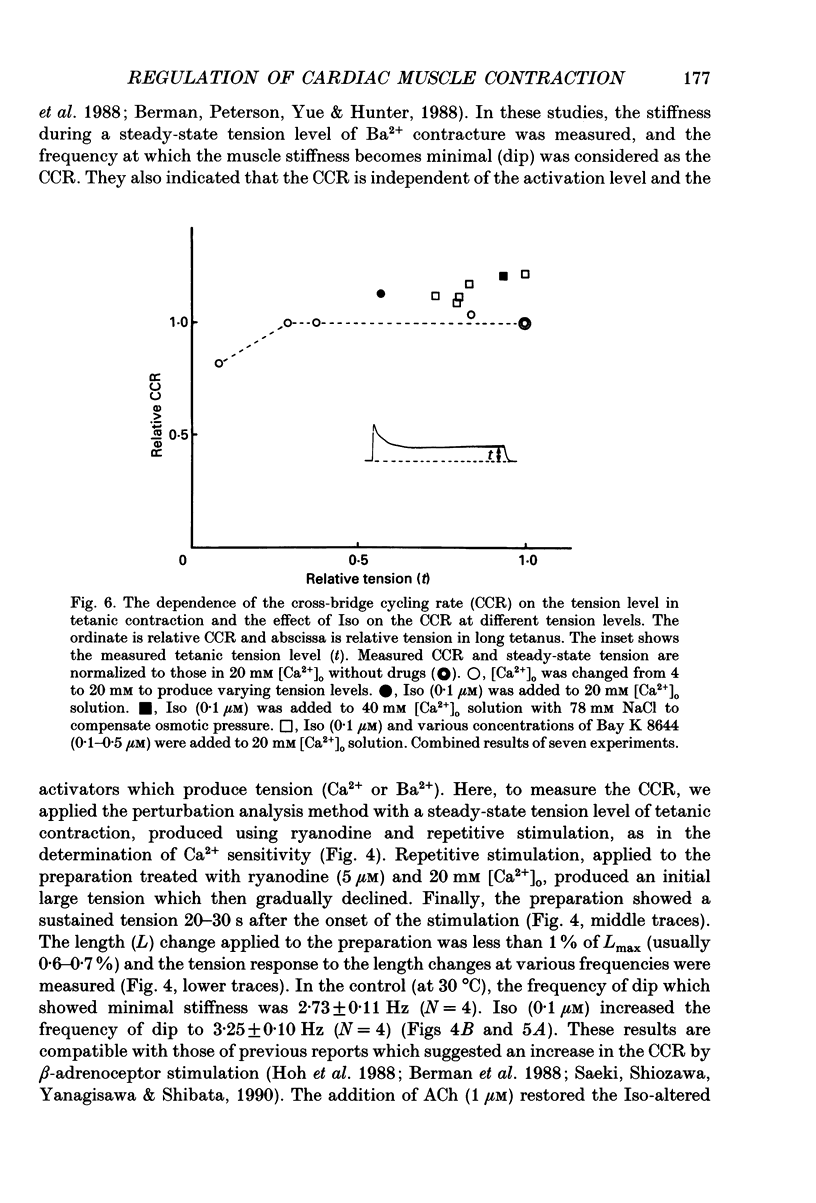
1. To clarify the mechanism which regulates the time course of twitch tension when beta- and muscarinic receptors are stimulated, intracellular Ca2+ transients, Ca2+ sensitivity of the contractile element and the cross-bridge cycling rate (CCR) were measured in ferret ventricular muscles. 2. Isoprenaline (Iso; 0.1 microM) increased peaks of Ca2+ transients measured with aequorin and tension, and abbreviated the time courses of both signals. Addition of acetylcholine (ACh; 0.01-1 microM) to the Iso-treated preparation dose dependently decreased the peaks of both signals and restored the time course of Ca2+ transients. However, the time course of tension was not recovered by the addition of ACh, and the relaxation time in particular, was further shortened by ACh. Carbachol (1 microM) applied to the Iso-treated preparation yielded similar results. 3. [Ca2+]i and tension at a quasi-steady level of tetanic contraction, which was produced by ryanodine (5 microM) and repetitive stimulation, were measured and Ca2+ sensitivity of the contractile element was estimated. Iso (0.1 microM) decreased the Ca2+ sensitivity and the addition of ACh (1 microM) completely recovered it to the control level. 4. In order to measure CCR, the perturbation analysis method was applied to steady-state tension of tetanic contraction. The CCR was not altered even when the tetanic tension level was decreased to 50% by decreasing [Ca2+]o. Iso (0.1 microM) slightly decreased the tetanic tension level and increased the CCR from 2.73 to 3.25 Hz. The effect of Iso was observed when the Iso-decreased tension was recovered by an increase in [Ca2+]i. The addition of ACh (1 microM) recovered the CCR which was increased by Iso, to the control level. Atropine (10 microM) blocked the effect of ACh, and carbachol (1 microM) restored the CCR increased by Iso to the control level. 5. The time course of Ca2+ transients, Ca2+ sensitivity and CCR were antagonistically regulated by beta- and muscarinic receptor stimulation, but the time course of tension did not parallel the changes in these parameters. Therefore, these results suggest that the time course of tension, particularly the relaxation time, is not determined by the time course of Ca2+ transients, Ca2+ sensitivity and the CCR, and that other factors might be involved in the regulation of the time course of tension when beta- and muscarinic receptors are stimulated.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kurihara S. Calcium transients in mammalian ventricular muscle. Eur Heart J. 1980;Suppl A:5–15. doi: 10.1093/eurheartj/1.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Berman M. R., Peterson J. N., Yue D. T., Hunter W. C. Effect of isoproterenol on force transient time course and on stiffness spectra in rabbit papillary muscle in barium contracture. J Mol Cell Cardiol. 1988 May;20(5):415–426. doi: 10.1016/s0022-2828(88)80133-0. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Kirby M. S., Orchard C. H., Roberts A. The negative inotropic effect of acetylcholine on ferret ventricular myocardium. J Physiol. 1988 Oct;404:613–635. doi: 10.1113/jphysiol.1988.sp017309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Blinks J. R. Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through alpha- and beta-adrenoceptors. Circ Res. 1988 Feb;62(2):247–265. doi: 10.1161/01.res.62.2.247. [DOI] [PubMed] [Google Scholar]
- Endoh M. Regulation of force and intracellular calcium transients by cyclic AMP generated by forskolin, MDL 17,043 and isoprenaline, and its modulation by muscarinic receptor agents: a novel mechanism for accentuated antagonism. Basic Res Cardiol. 1989;84 (Suppl 1):69–83. doi: 10.1007/BF02650348. [DOI] [PubMed] [Google Scholar]
- England P. J., Pask H. T., Mills D. Cyclic-AMP-dependent phosphorylation of cardiac contractile proteins. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:383–390. [PubMed] [Google Scholar]
- England P. J. Studies on the phosphorylation of the inhibitory subunit of troponin during modification of contraction in perfused rat heart. Biochem J. 1976 Nov 15;160(2):295–304. doi: 10.1042/bj1600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Titus L. Effects of cholinergic and adrenergic agonists on phosphorylation of a 165,000-dalton myofibrillar protein in intact cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):2111–2120. [PubMed] [Google Scholar]
- Hino N., Ochi R. Effect of acetylcholine on membrane currents in guinea-pig papillary muscle. J Physiol. 1980 Oct;307:183–197. doi: 10.1113/jphysiol.1980.sp013430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh J. F., Rossmanith G. H., Kwan L. J., Hamilton A. M. Adrenaline increases the rate of cycling of crossbridges in rat cardiac muscle as measured by pseudo-random binary noise-modulated perturbation analysis. Circ Res. 1988 Mar;62(3):452–461. doi: 10.1161/01.res.62.3.452. [DOI] [PubMed] [Google Scholar]
- Hongo K., Tanaka E., Kurihara S. Mechanism of the effects of acetylcholine on the contractile properties and Ca2+ transients in ferret ventricular muscles. J Physiol. 1993 Feb;461:185–199. doi: 10.1113/jphysiol.1993.sp019508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Halvorson H. R. Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned fibers of rabbit psoas muscle. Biophys J. 1991 Feb;59(2):329–342. doi: 10.1016/S0006-3495(91)82227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J. C. Combined inhibitory actions of acidosis and phosphate on maximum force production in rat skinned cardiac muscle. Pflugers Arch. 1991 Oct;419(3-4):310–318. doi: 10.1007/BF00371112. [DOI] [PubMed] [Google Scholar]
- Kurihara S., Allen D. G. Intracellular Ca++ transients and relaxation in mammalian cardiac muscle. Jpn Circ J. 1982 Jan;46(1):39–43. doi: 10.1253/jcj.46.39. [DOI] [PubMed] [Google Scholar]
- Kurihara S., Konishi M. Effects of beta-adrenoceptor stimulation on intracellular Ca transients and tension in rat ventricular muscle. Pflugers Arch. 1987 Aug;409(4-5):427–437. doi: 10.1007/BF00583798. [DOI] [PubMed] [Google Scholar]
- Kusuoka H., Weisfeldt M. L., Zweier J. L., Jacobus W. E., Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circ Res. 1986 Sep;59(3):270–282. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- Lindemann J. P., Jones L. R., Hathaway D. R., Henry B. G., Watanabe A. M. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983 Jan 10;258(1):464–471. [PubMed] [Google Scholar]
- Lindemann J. P., Watanabe A. M. Muscarinic cholinergic inhibition of beta-adrenergic stimulation of phospholamban phosphorylation and Ca2+ transport in guinea pig ventricles. J Biol Chem. 1985 Oct 25;260(24):13122–13129. [PubMed] [Google Scholar]
- MacKinnon R., Gwathmey J. K., Allen P. D., Briggs G. M., Morgan J. P. Modulation by the thyroid state of intracellular calcium and contractility in ferret ventricular muscle. Circ Res. 1988 Dec;63(6):1080–1089. doi: 10.1161/01.res.63.6.1080. [DOI] [PubMed] [Google Scholar]
- Marban E., Kusuoka H. Maximal Ca2+-activated force and myofilament Ca2+ sensitivity in intact mammalian hearts. Differential effects of inorganic phosphate and hydrogen ions. J Gen Physiol. 1987 Nov;90(5):609–623. doi: 10.1085/jgp.90.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIvor M. E., Orchard C. H., Lakatta E. G. Dissociation of changes in apparent myofibrillar Ca2+ sensitivity and twitch relaxation induced by adrenergic and cholinergic stimulation in isolated ferret cardiac muscle. J Gen Physiol. 1988 Oct;92(4):509–529. doi: 10.1085/jgp.92.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhfi H., Ventura-Clapier R. Dependence upon high-energy phosphates of the effects of inorganic phosphate on contractile properties in chemically skinned rat cardiac fibres. Pflugers Arch. 1988 Apr;411(4):378–385. doi: 10.1007/BF00587716. [DOI] [PubMed] [Google Scholar]
- Okazaki O., Suda N., Hongo K., Konishi M., Kurihara S. Modulation of Ca2+ transients and contractile properties by beta-adrenoceptor stimulation in ferret ventricular muscles. J Physiol. 1990 Apr;423:221–240. doi: 10.1113/jphysiol.1990.sp018019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K. P., England P. J. Phosphorylation of the inhibitory subunit of troponin and its effect on the calcium dependence of cardiac myofibril adenosine triphosphatase. FEBS Lett. 1976 Nov;70(1):11–16. doi: 10.1016/0014-5793(76)80716-8. [DOI] [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Holroyde M. J., Kranias E. G., Potter J. D., Solaro R. J. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem. 1982 Jan 10;257(1):260–263. [PubMed] [Google Scholar]
- Rossmanith G. H., Hoh J. F., Kirman A., Kwan L. J. Influence of V1 and V3 isomyosins on the mechanical behaviour of rat papillary muscle as studied by pseudo-random binary noise modulated length perturbations. J Muscle Res Cell Motil. 1986 Aug;7(4):307–319. doi: 10.1007/BF01753651. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Kawai M., Zhao Y. Comparison of crossbridge dynamics between intact and skinned myocardium from ferret right ventricles. Circ Res. 1991 Mar;68(3):772–781. doi: 10.1161/01.res.68.3.772. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Sagawa K., Suga H. Dynamic stiffness of cat heart muscle in Ba2+-induced contracture. Circ Res. 1978 Mar;42(3):324–333. doi: 10.1161/01.res.42.3.324. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Shiozawa K., Yanagisawa K., Shibata T. Adrenaline increases the rate of cross-bridge cycling in rat cardiac muscle. J Mol Cell Cardiol. 1990 Apr;22(4):453–460. doi: 10.1016/0022-2828(90)91480-u. [DOI] [PubMed] [Google Scholar]
- Tada M., Katz A. M. Phosphorylation of the sarcoplasmic reticulum and sarcolemma. Annu Rev Physiol. 1982;44:401–423. doi: 10.1146/annurev.ph.44.030182.002153. [DOI] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Repke D. I., Katz A. M. The stimulation of calcium transport in cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1974 Oct 10;249(19):6174–6180. [PubMed] [Google Scholar]
- Ten Eick R., Nawrath H., McDonald T. F., Trautwein W. On the mechanism of the negative inotropic effect of acetylcholine. Pflugers Arch. 1976 Feb 24;361(3):207–213. doi: 10.1007/BF00587284. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Kort A. A., Stern M. D., Lakatta E. G., Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegrad S. Regulation of cardiac contractile proteins. Correlations between physiology and biochemistry. Circ Res. 1984 Nov;55(5):565–574. doi: 10.1161/01.res.55.5.565. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Marban E., Wier W. G. Relationship between force and intracellular [Ca2+] in tetanized mammalian heart muscle. J Gen Physiol. 1986 Feb;87(2):223–242. doi: 10.1085/jgp.87.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]


