Abstract
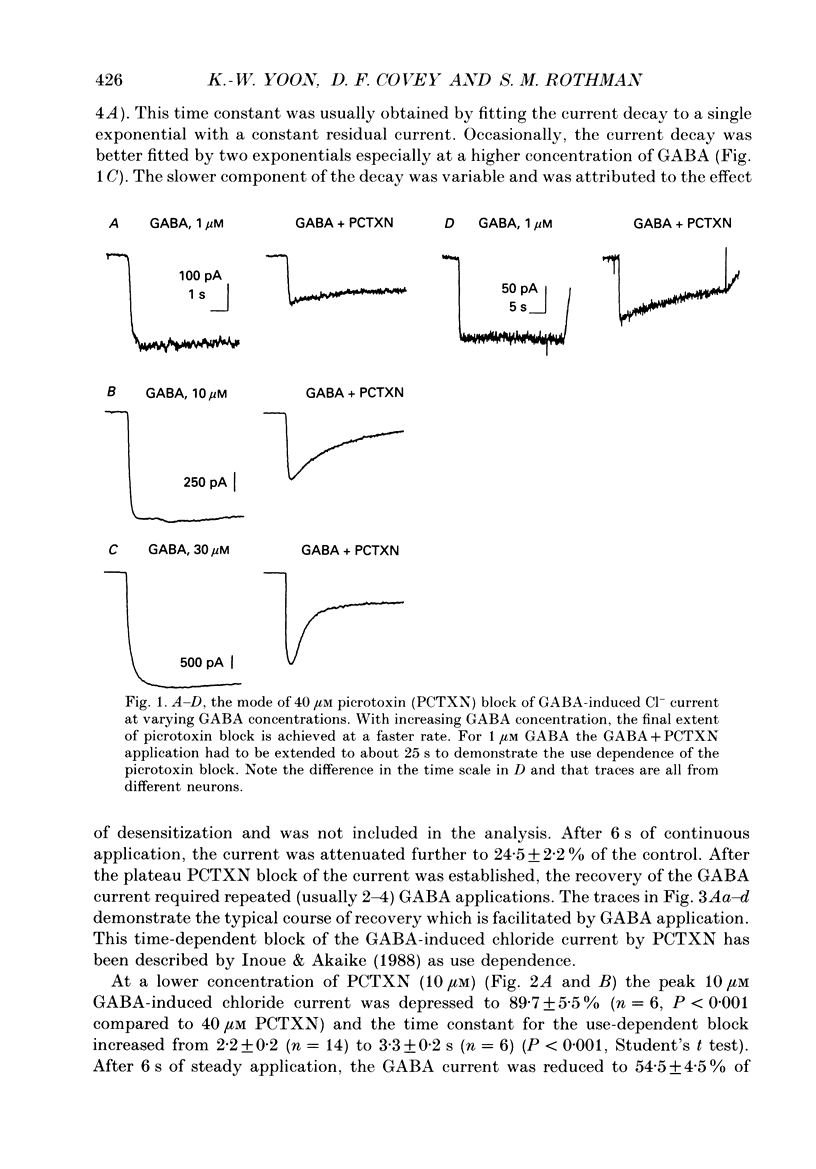
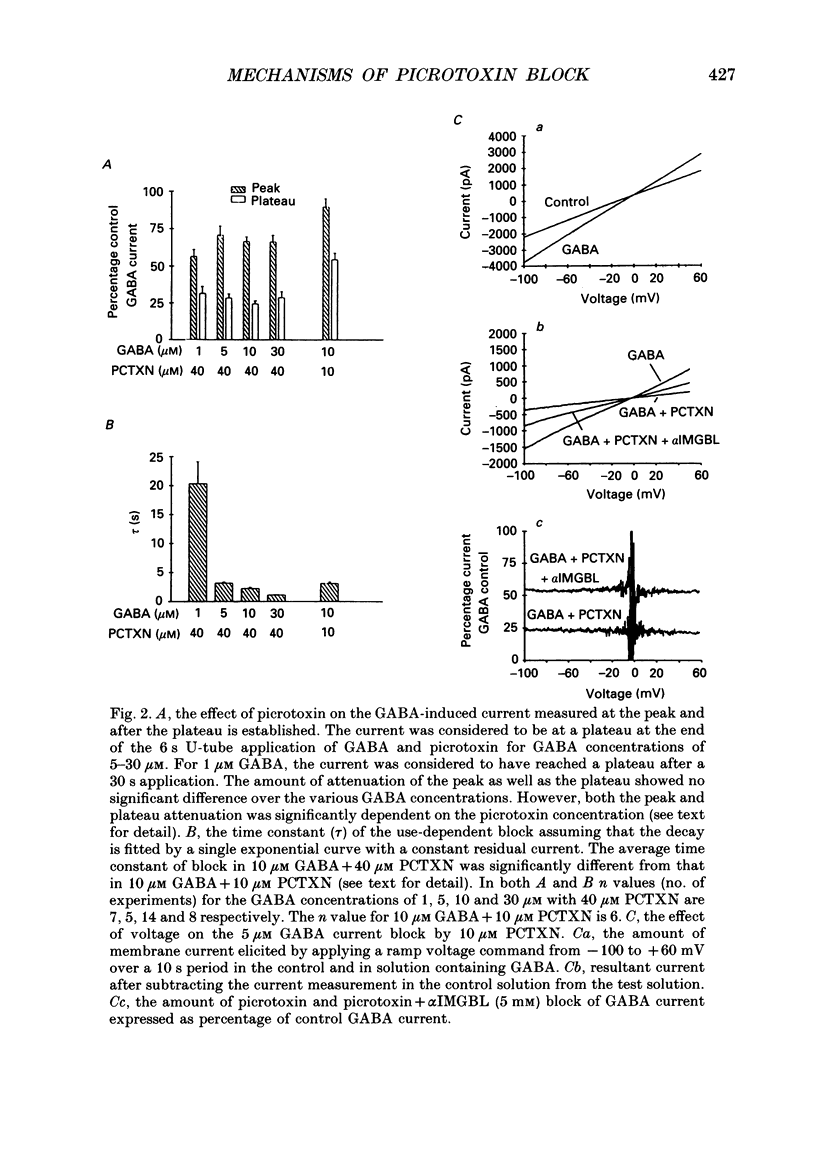
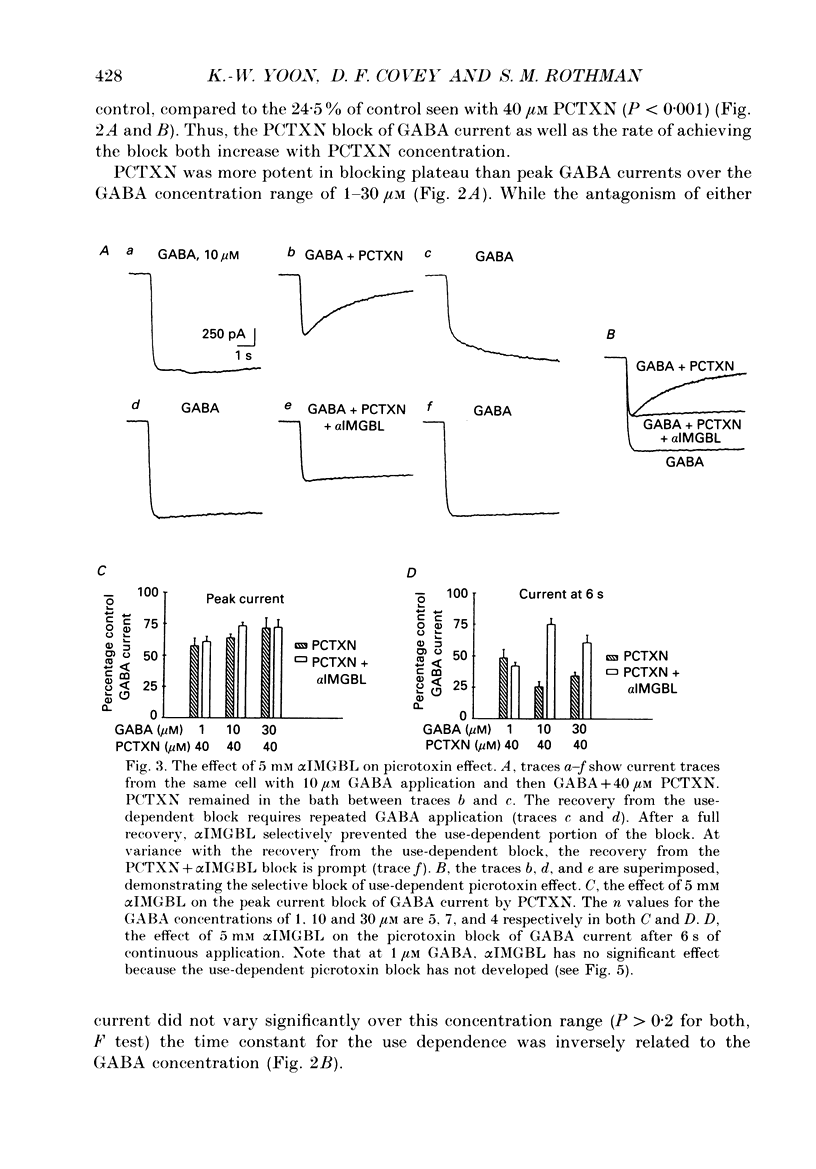
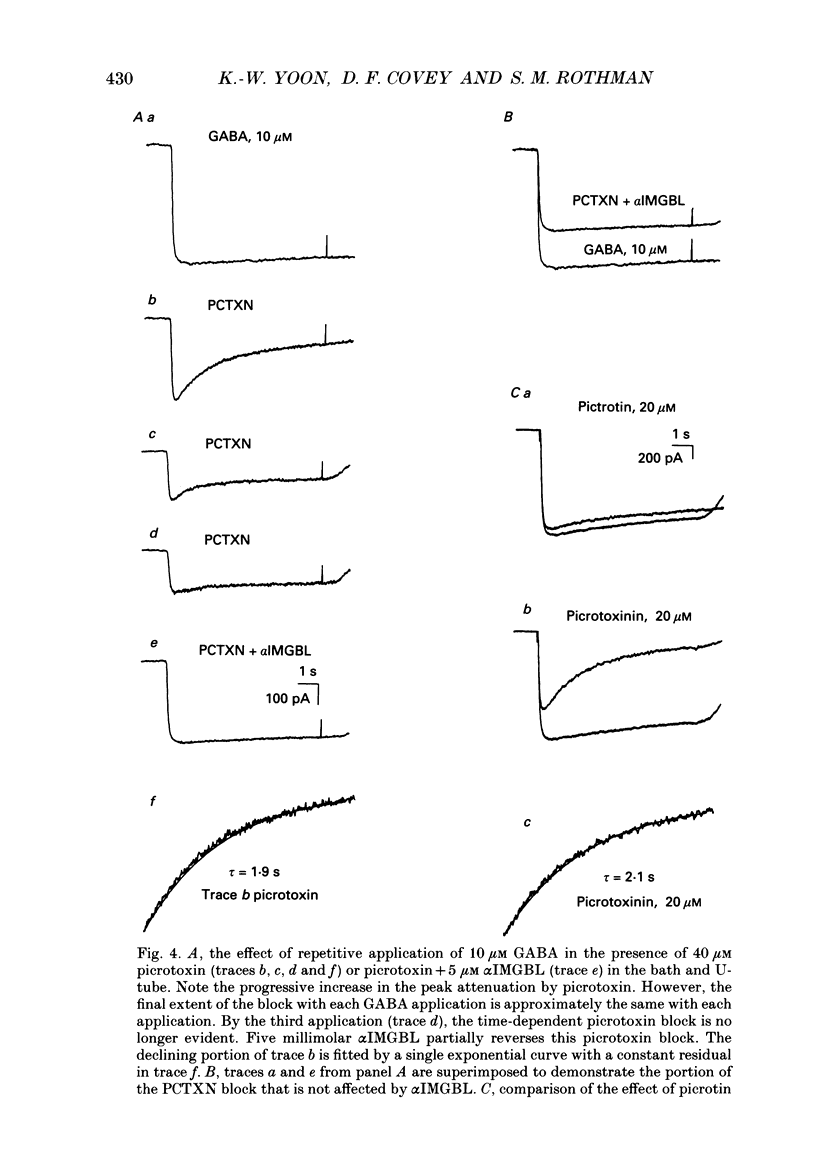
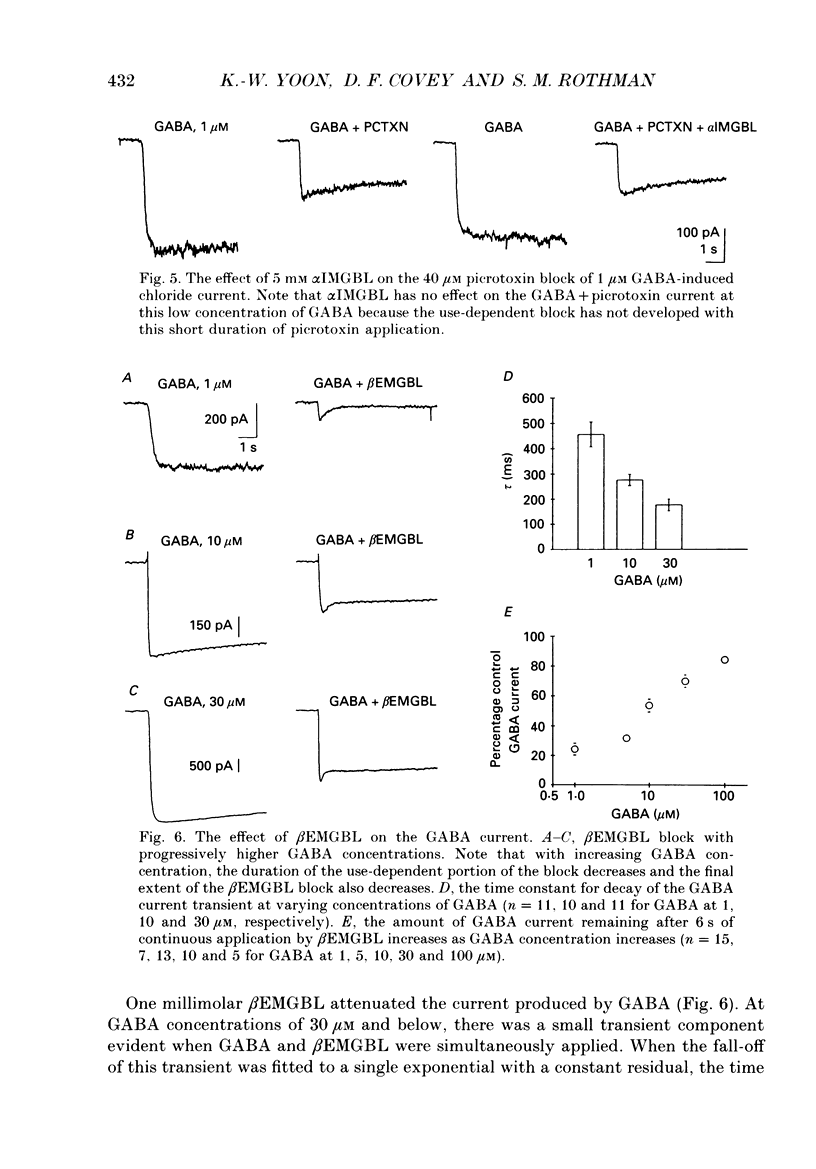
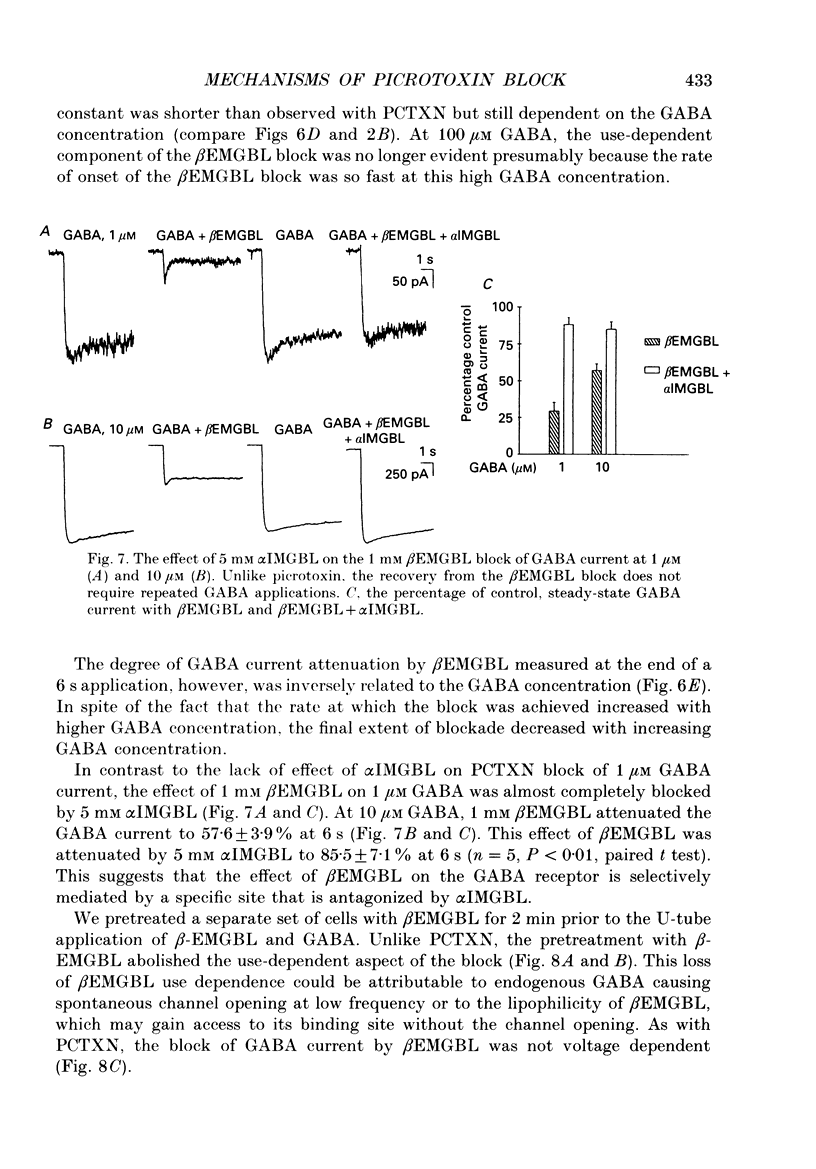
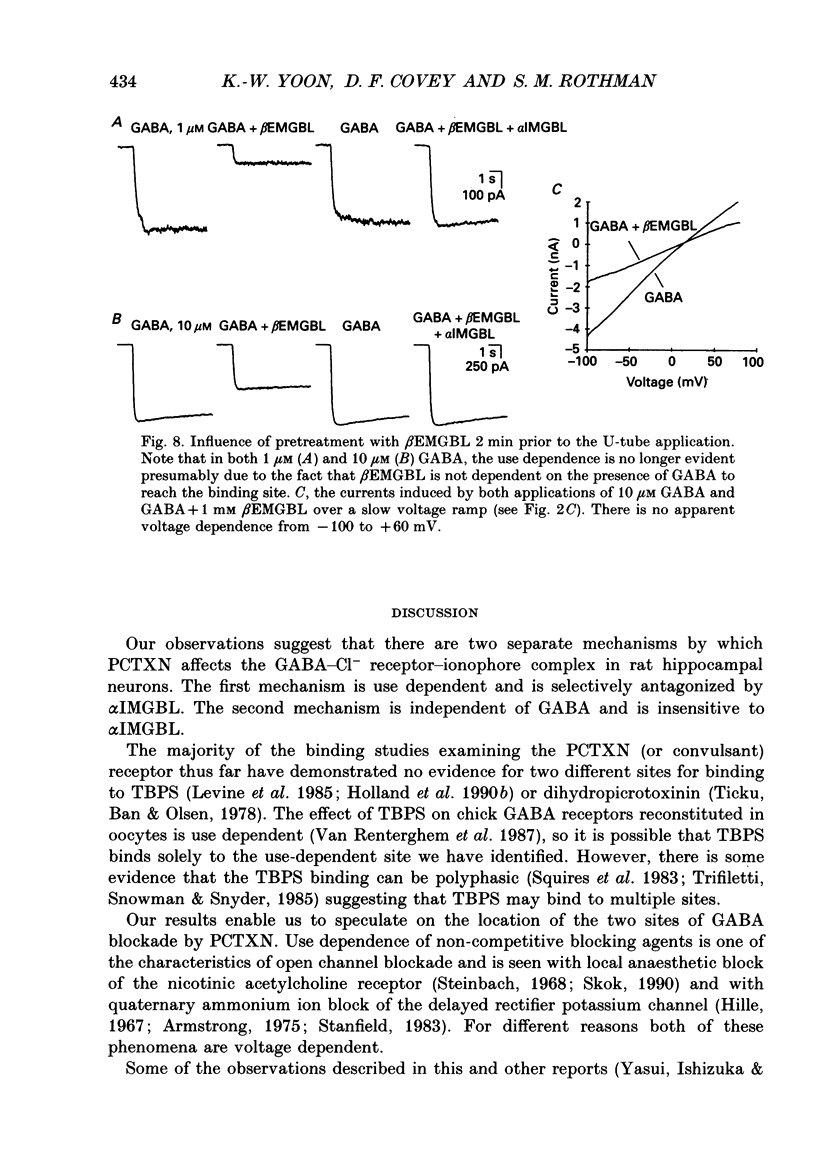
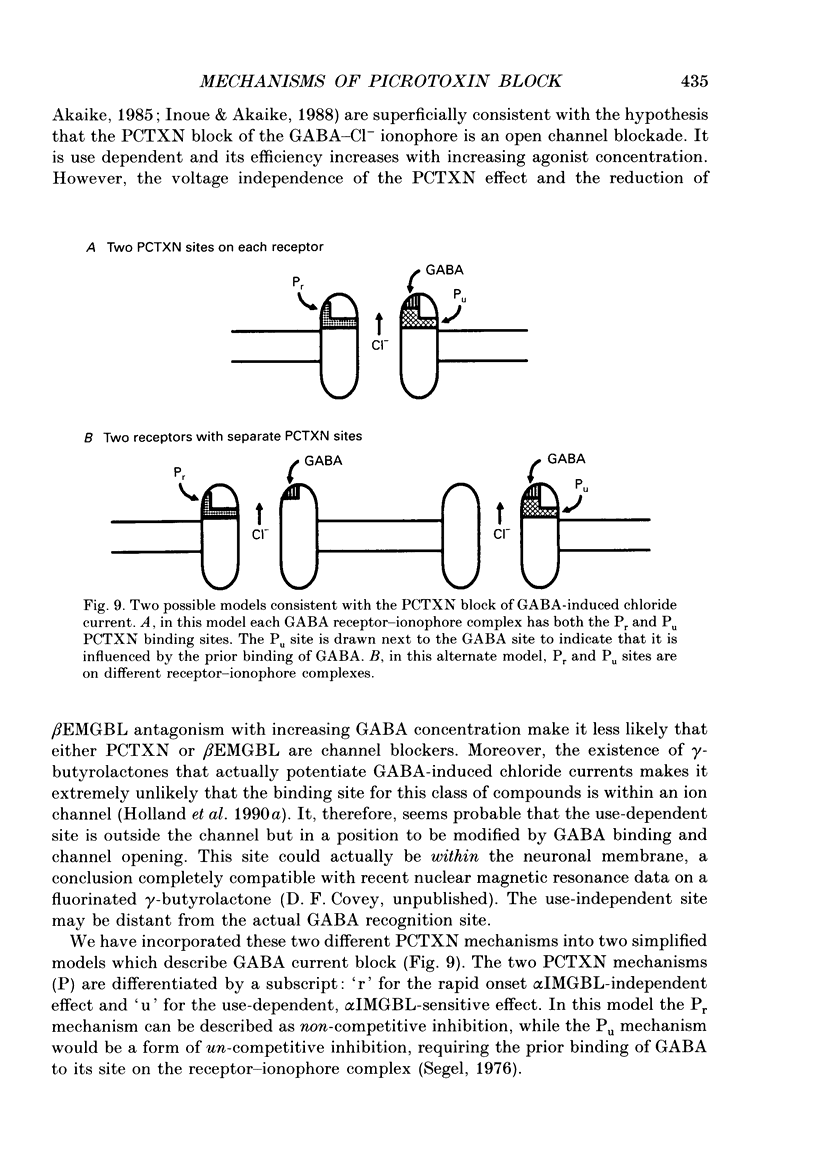
1. We have examined the effect of picrotoxin on GABA-induced currents in dissociated rat hippocampal neurons. In addition, we used the putative picrotoxin receptor antagonist, alpha-isopropyl-alpha-methyl-gamma-butyrolactone (alpha IMGBL), and the picrotoxin agonist, beta-ethyl-beta-methyl-gamma-butyrolactone (beta EMGBL) to explore the mechanisms of picrotoxin's interaction with the GABA-Cl- receptor-ionophore complex. 2. The picrotoxin block of GABA current was use dependent, suggesting that the site of picrotoxin block is exposed by the conformational change initiated by GABA binding to the receptor. 3. The alkyl-substituted butyrolactone antagonist, alpha IMGBL, selectively blocked the use-dependent mechanism of picrotoxin effect. After the apparent complete inhibition of the use-dependent effect, there was a residual picrotoxin effect that was independent of the time or concentration of GABA application. This indicates that the picrotoxin block of the GABA current is mediated by two different mechanisms. alpha IMGBL influences just one of these mechanisms. 4. The picrotoxin receptor agonist, beta EMGBL, exclusively blocked the GABA current in a use-dependent manner. Consistent with a use-dependent mechanism, the rate of onset of block increased with GABA concentration. Surprisingly, the fraction of GABA current block decreased with increasing GABA concentration. 5. These results suggest that the relationship of picrotoxin and gamma-butyrolactones with the GABA-Cl- receptor-ionophore is quite complex. They are consistent with at least two possible models of agonist-antagonist interactions. Both cases require different antagonist affinities for the various kinetic states of the GABA-Cl- receptor-ionophore. However, there is no need to require that either picrotoxin or beta EMGBL acts as an open channel blocker.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Hattori K., Oomura Y., Carpenter D. O. Bicuculline and picrotoxin block gamma-aminobutyric acid-gated Cl- conductance by different mechanisms. Experientia. 1985 Jan 15;41(1):70–71. doi: 10.1007/BF02005880. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M. Ionic pores, gates, and gating currents. Q Rev Biophys. 1974 May;7(2):179–210. doi: 10.1017/s0033583500001402. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Wise B. C., Vaccarino F., Guidotti A. gamma-Aminobutyric acid- and benzodiazepine-induced modulation of [35S]-t-butylbicyclophosphorothionate binding to cerebellar granule cells. J Neurosci. 1985 Sep;5(9):2432–2438. doi: 10.1523/JNEUROSCI.05-09-02432.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland K. D., Ferrendelli J. A., Covey D. F., Rothman S. M. Physiological regulation of the picrotoxin receptor by gamma-butyrolactones and gamma-thiobutyrolactones in cultured hippocampal neurons. J Neurosci. 1990 Jun;10(6):1719–1727. doi: 10.1523/JNEUROSCI.10-06-01719.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland K. D., McKeon A. C., Covey D. F., Ferrendelli J. A. Binding interactions of convulsant and anticonvulsant gamma-butyrolactones and gamma-thiobutyrolactones with the picrotoxin receptor. J Pharmacol Exp Ther. 1990 Aug;254(2):578–583. [PubMed] [Google Scholar]
- Inoue M., Akaike N. Blockade of gamma-aminobutyric acid-gated chloride current in frog sensory neurons by picrotoxin. Neurosci Res. 1988 Jun;5(5):380–394. doi: 10.1016/0168-0102(88)90024-7. [DOI] [PubMed] [Google Scholar]
- Klunk W. E., Covey D. F., Ferrendelli J. A. Anticonvulsant properties of alpha, gamma, and alpha, gamma-substituted gamma-butyrolactones. Mol Pharmacol. 1982 Sep;22(2):438–443. [PubMed] [Google Scholar]
- Klunk W. E., Covey D. F., Ferrendelli J. A. Structure-activity relationships of alkyl-substituted gamma-butyrolactones and succinimides. Mol Pharmacol. 1982 Sep;22(2):444–450. [PubMed] [Google Scholar]
- Macdonald R. L., Rogers C. J., Twyman R. E. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1989 Mar;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska M. D., Demirgören S., Spivak C. E., London E. D. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990 Aug 27;526(1):143–146. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Schwartz R. D. Pregnenolone-sulfate: an endogenous antagonist of the gamma-aminobutyric acid receptor complex in brain? Brain Res. 1987 Feb 24;404(1-2):355–360. doi: 10.1016/0006-8993(87)91394-1. [DOI] [PubMed] [Google Scholar]
- Mathers D. A., Wang Y. H. Effect of agonist concentration on the lifetime of GABA-activated membrane channels in spinal cord neurons. Synapse. 1988;2(6):627–632. doi: 10.1002/syn.890020608. [DOI] [PubMed] [Google Scholar]
- Newland C. F., Cull-Candy S. G. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat. J Physiol. 1992 Feb;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polc P. Electrophysiology of benzodiazepine receptor ligands: multiple mechanisms and sites of action. Prog Neurobiol. 1988;31(5):349–423. doi: 10.1016/0301-0082(88)90014-7. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Casida J. E., Richardson M., Saederup E. [35S]t-butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to gamma-aminobutyric acid-A and ion recognition sites. Mol Pharmacol. 1983 Mar;23(2):326–336. [PubMed] [Google Scholar]
- Stanfield P. R. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev Physiol Biochem Pharmacol. 1983;97:1–67. doi: 10.1007/BFb0035345. [DOI] [PubMed] [Google Scholar]
- Steinbach A. B. A kinetic model for the action of xylocaine on receptors for acetylcholine. J Gen Physiol. 1968 Jul;52(1):162–180. doi: 10.1085/jgp.52.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticku M. K., Ban M., Olsen R. W. Binding of [3H]alpha-dihydropicrotoxinin, a gamma-aminobutyric acid synaptic antagonist, to rat brain membranes. Mol Pharmacol. 1978 May;14(3):391–402. [PubMed] [Google Scholar]
- Trifiletti R. R., Snowman A. M., Snyder S. H. Anxiolytic cyclopyrrolone drugs allosterically modulate the binding of [35S]t-butylbicyclophosphorothionate to the benzodiazepine/gamma-aminobutyric acid-A receptor/chloride anionophore complex. Mol Pharmacol. 1984 Nov;26(3):470–476. [PubMed] [Google Scholar]
- Twyman R. E., Rogers C. J., Macdonald R. L. Intraburst kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1990 Apr;423:193–220. doi: 10.1113/jphysiol.1990.sp018018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Renterghem C., Bilbe G., Moss S., Smart T. G., Constanti A., Brown D. A., Barnard E. A. GABA receptors induced in Xenopus oocytes by chick brain mRNA: evaluation of TBPS as a use-dependent channel-blocker. Brain Res. 1987 Apr;388(1):21–31. doi: 10.1016/0169-328x(87)90017-9. [DOI] [PubMed] [Google Scholar]
- Weiss D. S., Magleby K. L. Gating scheme for single GABA-activated Cl- channels determined from stability plots, dwell-time distributions, and adjacent-interval durations. J Neurosci. 1989 Apr;9(4):1314–1324. doi: 10.1523/JNEUROSCI.09-04-01314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman B. A., Burke T. R., Jr, Rice K. C., Skolnick P. Alkyl-substituted gamma-butyrolactones inhibit [35S]TBPS binding to a GABA linked chloride ionophore. Eur J Pharmacol. 1984 Oct 1;105(1-2):195–196. doi: 10.1016/0014-2999(84)90669-1. [DOI] [PubMed] [Google Scholar]
- Yamada K. A., Dubinsky J. M., Rothman S. M. Quantitative physiological characterization of a quinoxalinedione non-NMDA receptor antagonist. J Neurosci. 1989 Sep;9(9):3230–3236. doi: 10.1523/JNEUROSCI.09-09-03230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui S., Ishizuka S., Akaike N. GABA activates different types of chloride-conducting receptor-ionophore complexes in a dose-dependent manner. Brain Res. 1985 Sep 30;344(1):176–180. doi: 10.1016/0006-8993(85)91206-5. [DOI] [PubMed] [Google Scholar]
- Yoon K. W., Canney D. J., Covey D. F., Rothman S. M. Modulation of the picrotoxin receptor by fluorinated ethyl, methyl-butyrolactones. J Pharmacol Exp Ther. 1990 Oct;255(1):248–255. [PubMed] [Google Scholar]


