Abstract
Mammary anlagen are formed in the embryo as a derivative of the epidermis, a process that is controlled by Lef-1 and therefore possibly by β-catenin. To investigate the role of β-catenin signaling in mammary alveolar epithelium, we have stabilized endogenous β-catenin in differentiating alveolar epithelium through the deletion of exon 3 (amino acids 5–80) of the β-catenin gene. This task was accomplished in mice carrying a floxed β-catenin gene and a Cre transgene under control of the mammary-specific whey acidic protein (WAP) gene promoter or the mouse mammary tumor virus-long terminal repeat (MMTV-LTR). Stabilized β-catenin was obtained during the first pregnancy, and its presence resulted in the dedifferentiation of alveolar epithelium followed by a transdifferentiation into epidermal and pilar structures. Extensive squamous metaplasia, but no adenocarcinomas, developed upon β-catenin activation during pregnancy and persisted throughout involution. These data demonstrate that the activation of β-catenin signaling induces a program that results in loss of mammary epithelial cell differentiation and induction of epidermal structures.
Keywords: mammary gland‖cell identity‖Wnt-signaling‖Cre‖dedifferentiation
In addition to its role in cell–cell adhesion, β-catenin is also an integral part of the Wnt pathway, which has been linked to developmental processes including cell fate specification, polarity, and migration (1). Upon binding of Wnt proteins to the transmembrane Frizzled receptor, a signaling cascade is activated that leads to the stabilization of β-catenin and its interaction with the lymphoid enhancer transcription factors (LEFs) and T cell factors (Tcfs), followed by translocation of the entire complex to the nucleus (2, 3). The roles of β-catenin in development have been investigated through genetic loss- and gain-of-function experiments (4–7), which demonstrated the importance of β-catenin in the determination of the anterior–posterior axis and mesoderm structures during fetal development, and its role in the determination of epidermal cell fate. A role for Wnt/β-catenin signaling in fetal and adult mammary development and tumorigenesis has been suggested (8, 9). Most notably, mammary anlagen failed to be formed in Lef-1 null mice (8). In addition, expression of an N-terminally truncated β-catenin isoform under the control of a MMTV-LTR resulted in aberrant alveolar budding, precocious alveolar development, and the formation of mammary adenocarcinomas (10).
We hypothesize that the identity and differentiation of mammary alveolar epithelium depends on balanced β-catenin/LEF-1 signaling. If β-catenin signaling is the primary determinant that maintains the specification of alveolar epithelium, activation of β-catenin may cause a reversion of secretory epithelium into epidermis. To test this hypothesis, we have used Catnb+lox(ex3) mice (11), which generate a stabilized form of β-catenin upon Cre-mediated excision of exon 3 (amino acid 5–80). Deletion in mammary epithelium was achieved through the use of WAP-Cre transgenic mice that carry the Cre gene under control of the whey acidic protein (WAP) gene promoter or the MMTV-LTR (12). The WAP gene promoter is active in mammary alveolar epithelium transiently during estrus and during pregnancy (13, 14), and MMTV-LTR is active in ductal epithelium during puberty and in alveolar epithelium throughout pregnancy (15, 16).
Materials and Methods
Generation of Mammary-Specific Constitutively Activate β-Catenin.
The floxed β-catenin mice and the WAP-Cre and MMTV-Cre transgenic mice have been described (11, 12). Mice containing one wild-type β-catenin allele and one conditional allele (Catnb+/lox(ex3)) were mated with the WAP-Cre or MMTVCre mice to generate Catnb+/lox(ex3):Tg:WAPcre mice or Catnb+/lox(ex3):Tg:MMTVcre mice. Females with this genotype were mated to facilitate WAP/MMTV-Cre-mediated deletion of exon 3 of the β-catenin gene (referred to as Catnb+/Δex3:WAPcre mice or Catnb+/Δex3:MMTVcre). For analyses during pregnancy, the left no. 4 mammary gland was removed and divided into three parts; one for histology, one for Western blotting, and one for immunohistochemistry. The contralateral gland was removed at lactation.
Abs and Western Blotting.
The Abs were obtained from the following sources: mouse monoclonal anti-β-catenin (Transduction Laboratories, Lexington, KY), rabbit polyclonal anti-Stat5a (Stat, signal transducer and activator of transcription) (17), mouse anti-actin mAb (Chemicon), rabbit polyclonal anti-α-catenin (Sigma), rabbit polyclonal anti-keratin 1 (Babco, Richmond, CA), and rabbit polyclonal anti-keratin 5 (Babco). Rabbit polyclonal anti-Na-K-Cl cotransporter 1 (NKCC1) and rabbit polyclonal anti-Npt2b (Npr2b = Na-Pi cotransporter type II b) were the kind gifts from Jim Turner, National Institute of Dental and Craniofacial Research, National Institutes of Health and Jurg Biber, Department of Physiology, University of Zurich, Switzerland, respectively. FITC-conjugated anti-mouse (Alexafluor 488) and Texas Red-conjugated anti-rabbit (Alexafluor 594) secondary Abs (Molecular Probes) were used for immunohistochemistry and horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit secondary Abs were used for Western blotting (Transduction Laboratories).
Protein was isolated from mammary tissue as described (17). A total of 40 μg of protein was separated by electrophoresis in Tris-glycine gels (Invitrogen). Membranes were blocked overnight at 4°C in TBST (Tris-buffered saline, pH 7.4, with 0.05% Tween 20) containing 5% (wt/vol) nonfat dried milk. Primary Abs were incubated with the membranes for 1 h at room temperature. Subsequently, membranes were incubated with an HRP-linked anti-mouse or anti-rabbit secondary Ab for 30 min at room temperature. Specifically bound Ab was visualized by using an enhanced chemiluminescence procedure (Supersignal, Pierce).
Histology and Immunohistochemistry.
Mammary epithelium was isolated from Catnb+/Δex3 mice and fixed in Tellyesniczky's fixative for 4 h. After fixation, samples were dehydrated, embedded in paraffin, and sectioned at 5 μm. For histology, sections were stained with hematoxylin and eosin. For immunohistochemistry, primary Abs (keratin 1, 1:200, keratin 5, 1:200, α-catenin, 1:100; β-catenin, 1:100; Npt2b, 1:100; and NKCC1, 1:1,000) were applied to the sections and incubated at 37°C for 1 h. Sections were subsequently incubated with fluorescent-conjugated anti-mouse and anti-rabbit secondary Abs at 37°C for 1 h.
Results and Discussion
Stabilization of β-Catenin in Mammary Alveolar Epithelium.
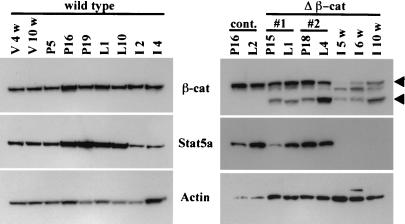
Catnb+/lox(ex3):Tg:WAPcre mice were generated, and we followed the deletion of exon 3 in mammary tissue in these mice (referred to as Catnb+/Δex3:WAPcre mice) through the analysis of β-catenin protein. In wild-type mice, β-catenin was present throughout puberty, pregnancy, and lactation (Fig. 1 Left) and was localized on the basolateral membrane of the mammary epithelial cells (see Fig. 4 A and D). Truncated β-catenin was detected in Catnb+/Δex3:WAPcre mice at day 15 of pregnancy (P15) (Fig. 1 Right, lower arrowhead). A biopsy taken from the same mouse at day 1 of lactation (L1) demonstrated a similar extent of excision (Fig. 1 Right #1). However, the amount of truncated β-catenin increased by day 4 of lactation (L4) (Fig. 1 Right #2). High levels of truncated β-catenin were also detected several weeks after involution (Fig. 1 Right).
Figure 1.
Western blot analyses of wild-type mice and mice expressing stabilized β-catenin (Catnb+/Δex3). β-catenin, Stat5a, and actin protein levels during wild-type mammary gland development (Left) and Catnb+/Δex3 mice (Right). The arrowheads point to the wild-type (Upper) and truncated (Lower) β-catenin forms, respectively. (Right) The lower bars (#1 and #2) indicate tissue derived from the two respective time points of the same mouse. All mice were mated at 12 wk of age. V, virgin; P, pregnancy; L, lactation; I, involution; V 4w, virgin 4-wk-old; V 10w, virgin 10-wk-old; P5, P16, and P19, pregnancy day 5, 16, and 19; L1 and L12, lactation day 1 and day 12; I2 and I4, involution day 2 and 4; cont., control; and Δβ-cat, Catnb+/Δex3:WAPCremice.
Figure 4.
Loss of Npt2b and NKCC1 expression and cytoplasmic accumulation of β-catenin in Catnb+/Δex3 WAPcre mice at lactation day 1. (A–C) Immunofluorescent staining by using Npt2b (red) and β-catenin (green) primary Abs. (D–F) Immunofluorescent staining using NKCC1 (red) and β-catenin (green) primary Abs. (A) Wild-type alveoli showing apical localization of Npt2b (white arrowhead) and basolateral localization of β-catenin. (B) An alveolus showing loss of apical Npt2b (white arrow) adjacent to alveoli exhibiting apical Npt2b similar to wild-type. (C) Cytoplasmic accumulation of β-catenin (white circle) and loss of detectable apical Npt2b. (D) Wild-type alveoli exhibiting colocalization (orange) of NKCC1 and β-catenin in the basolateral membrane. (E) Loss of alveolar NKCC1 expression (white circle) but normal membranous expression of β-catenin. (F) Loss of alveolar NKCC1 expression (white arrowhead) and cytoplasmic accumulation of β-catenin.
Transdifferentiation of Mammary Alveolar Epithelium into Epidermis.
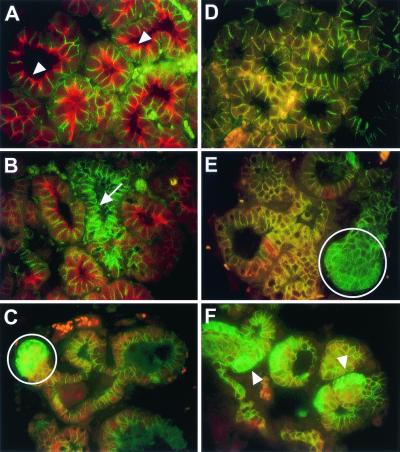
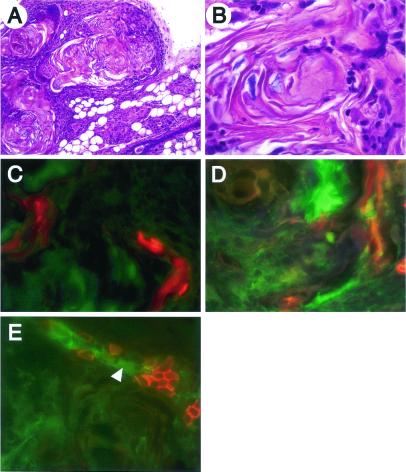
Histological analysis of mammary tissue from Catnb+/Δex3:WAPcre mice at day 15 of pregnancy revealed the presence of differentiating alveoli; hyperplastic structures with squamous metaplasia; and unusual squamous cysts (Fig. 2 A–C). Many terminal ductal units were nodular, and the individual alveolar units were lined by squamous cells with either central keratin debris or islands filled with cells containing translucent nuclei (“ghost cells”) (Fig. 2C). These keratinized structures tended to be asymmetrical, with a thicker layer of squamous cells at one edge. The squamous cells undergo maturation frequently ending with a granular layer with keratohyalin granules similar to the epidermis. As the lesions became more complex, the ghost cells became compressed and the cytoplasm did not readily stain with eosin, suggesting a very dense keratin similar to hair matrix. Some cross sectional profiles of these lesions were symmetrical with condensed laminar matrix that closely resembled pilar structures. This is suggestive of differentiation toward epidermis and hair and is referred to as “pilar differentiation.” We also observed extensive cell proliferation at P15 in Catnb+/Δex3:WAPcre mice, but not in wild-type controls (data not shown), which correlated well with the pattern of expression previously observed in the epidermis (18). At day 1 of lactation, some alveoli had undergone differentiation as evidenced by their secretory activity (Fig. 2 D–F), which was in agreement with increased Stat5 levels (Fig. 1 Right) and the ability of dams to lactate. However, the number of nodular structures filled with ghost cells (Fig. 2F), keratin (see Fig. 2H), and pilar differentiation (see Fig. 2I) had increased. The observed lesions were more severe after multiple pregnancies and dams were unable to lactate (data not shown). There was little evidence of normal alveoli, and many of the epithelial structures had expanded and were fully keratinized. The cysts had central keratin that was loosely laminated and was organized as individual, discrete swirls again reflecting the tendency toward pilar differentiation. The hyperplastic nodular structures and keratinized cysts failed to undergo weaning-induced regression and were retained after involution (Fig. 2 G–I). This can be explained by increased cell proliferation in the outer layer of the cysts and the lack of significant apoptosis (data not shown).
Figure 2.
Transdifferentiation of mammary alveolar epithelia to epidermal structures. (A) At pregnancy day 15, the gland was heterogeneously differentiated. Evidence of normal alveolar structures (B) and hyperproliferative epidermal cysts containing ghost cells (C). (D) At lactation day 1, the transdifferentiated lesions were more widespread. (E) Evidence of apparently normal secreting alveoli and (F) hyperproliferative epidermal cysts containing ghost cells. (G) Ten weeks after involution, we preferentially detected squamous metaplasias. (H) Cells were consistently hyperplastic and the center of the nodule was keratinized. (I) Evidence of the epidermal appearance of these structures.
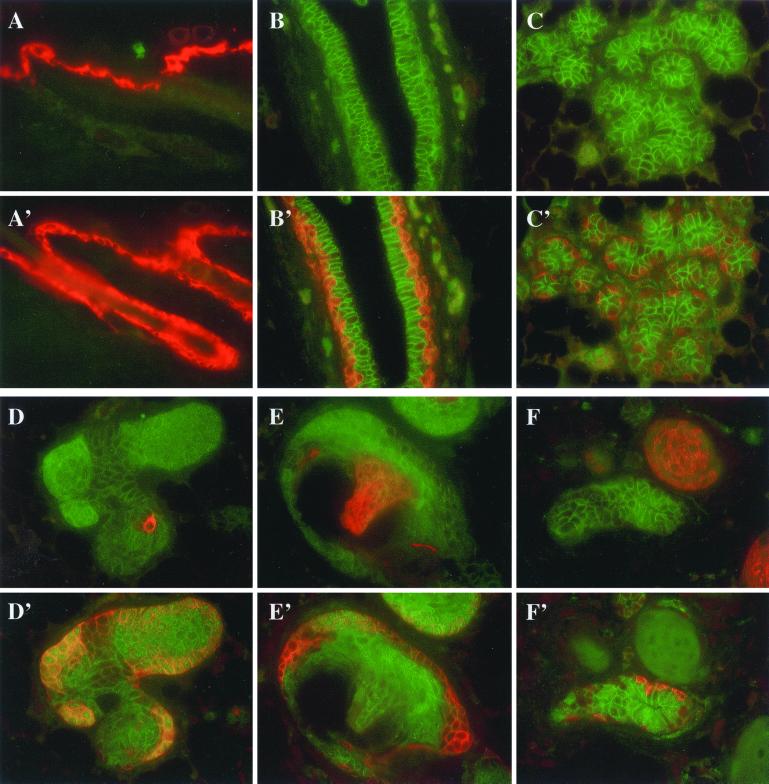
To verify the transdifferentiation into epidermis, the presence of keratins K1 and K5 was investigated (Fig. 3). In wild-type epidermis, K1 was found in the spinous layer (Fig. 3A), and K5 staining was observed preferentially in the basal layer and outer root sheath of hair follicles (Fig. 3A′). K1 was not found in normal mammary epithelium (Fig. 3 B and C), and K5 was found in the myoepithelium surrounding ducts and alveoli (Fig. 3 B′ and C′). K1 and K5 were detected in hyperplastic structures and squamous cells upon activation of β-catenin (Fig. 3 D–F). Although K5 was preferentially found in the outer cell layers (Fig. 3 D′–F′), K1 was preferentially located in the center of nodules and pilar structures (Fig. 3 D–F). These expression patterns resembled those seen during epidermal differentiation (19). Our results demonstrate that stabilized β-catenin induces transdifferentiation of alveolar epithelium into epidermal cells and squamous metaplasias.
Figure 3.
Keratin 1 (K1) and 5 (K5) are expressed in mammary epithelium transdifferentiated into epidermal structures. (A and A′) Immunofluorescent staining for K1 (red) and K5 (red) in wild-type epidermis. (B–F and B′–F′) Immunofluorescent staining for K1 (red) and K5 (red) and β-catenin (green) in Catnb+/Δex3 WAPcre mice at pregnancy day 15. K1 was detected in the spinous layer only (A) and K5 in the spinous and basal layers (A′). In normal ductal and alveolar structures, no K1 (B and C) was expressed and K5 was found in myoepithelial cells (B′ and C′). In nodules, β-catenin started to accumulate and cells in the center started to express K1 (D). K5 was only found in the outer layers (D′ and E′). K1 was detected in the pilar part (E). The nodule next to normal mammary epithelium expressed K1 but not K5 (F and F′). K5 was detected in myoepithelial cells (F′).
Alveolar Differentiation Is Lost On Stabilization of β-Catenin.
Mammary alveolar epithelium undergoes proliferation and differentiation during pregnancy, and the presence of high levels of Stat5 is a hallmark of functionally differentiated alveolar epithelia. Stat5 levels in wild-type mice increased during pregnancy, remained high during lactation and decreased during involution (Fig. 1 Left). The presence of truncated β-catenin in the Catnb+/Δex3 mice did not grossly alter Stat5 levels during the first pregnancy (Fig. 1 Right). However, no Stat5 was detected after involution in Catnb+/Δex3:WAPcre mice (Fig. 1 Right). This suggests that the stabilization of β-catenin leads to the loss of Stat5, possibly through the process of transdifferentiation to cell types that do not normally express Stat5.
Loss of differentiation was investigated on a cellular level by using β-catenin together with markers that characterize normal mammary epithelium. We determined the expression of NKCC1, which is normally expressed in ductal cells at the basolateral membrane (Fig. 4D), and Npt2b, which is present on the apical membrane of secretory alveolar epithelium (Fig. 4A, arrow). Because mammary tissue from Catnb+/Δex3:WAPcre mice during lactation contained normal epithelium and structures representing different stages of transdifferentiation and squamous metaplasia, it was possible to compare the expression of these markers throughout the progression of the lesions. Stabilization of β-catenin resulted in the loss of apical Npt2b (Fig. 4B, arrow) and basolateral NKCC1 (Fig. 4E, circle), events that occurred before significant accumulation of β-catenin (Fig. 4 C and F, arrowhead). This demonstrates that an early effect of stabilized β-catenin in mammary secretory cells is the loss of their normal epithelial characteristics.
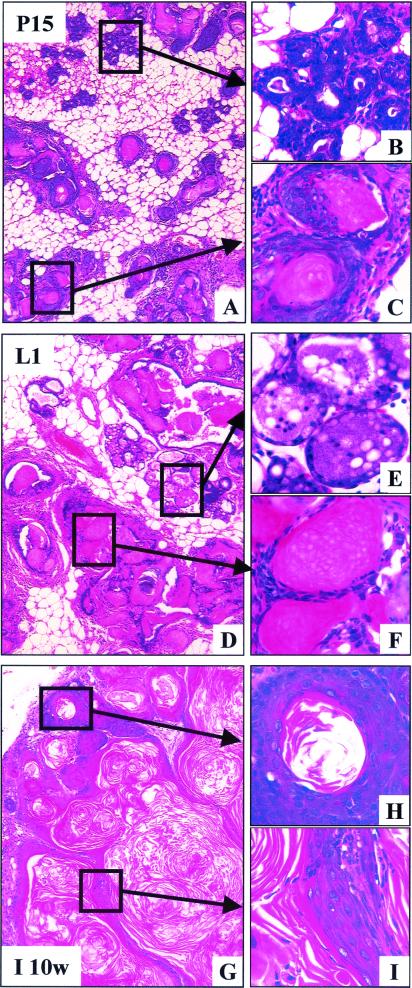
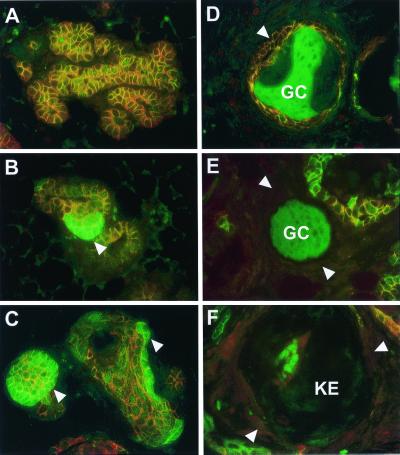
Because β-catenin forms a complex with E-cadherin and α-catenin, we reasoned that stabilization of β-catenin may result in alterations of E-cadherin and α-catenin localization. However, despite the accumulation of β-catenin (Fig. 5B) there was no evidence of alterations in the membranous localization of α-catenin (Fig. 5B) or E-cadherin (data not shown). Immunohistochemical analyses by using α- and β-catenin Abs revealed different types of lesion, which may represent the progressive nature of the transdifferentiation process in Catnb+/Δex3:WAPcre mice (Fig. 5). Based on the expression patterns of α- and β-catenin, we defined several distinct stages of transdifferentiation. In areas that maintained apparent normal alveolar structures, membranous localization of α- and β-catenin was observed (Fig. 5A). This pattern was similar to that seen in wild-type mice (data not shown). The first stage of transdifferentiation (stage I; Fig. 5B) was characterized by the local accumulation of β-catenin but normal membranous localization of α-catenin. During the next stage (stage II; Fig. 5C), the formation of cyst-like structures that possessed small lumina was observed. These structures exhibited accumulation of β-catenin and normal membranous localization of α-catenin. At stage III (Fig. 5D), membranous localization of α- and β-catenin was accompanied by the accumulation of ghost cells in the lumina. This feature was followed by a loss of α- and β-catenin staining in stage IV (Fig. 5E). The lumina of these structures were filled with ghost cells. In stage V no β-catenin staining was observed, and keratin-like deposits were apparent in the lumen (Fig. 5F). The frequency of stage I decreased as the mice went through pregnancy and concomitantly the number of stage V structures increased (data not shown).
Figure 5.
Progression of transdifferentiation in Catnb+/Δex3 WAPcre mice. (A) Area of apparently normal alveoli with normal membranous localization of α- (red) and β- (green) catenin. The structures appear orange. (B) Stage I: Normal membranous localization of α-catenin, accompanied by local accumulation of β-catenin (arrowhead). (C) Stage II: Normal membranous localization of α-catenin and the formation of cyst-like structures that exhibit accumulation of β-catenin (arrowheads). (D) Stage III: Cysts exhibiting membranous localization of α- and β-catenin (arrowhead), accompanied by the accumulation of ghost cells (GC) in the lumen (nonspecific green staining). (E) Stage IV: Complete loss of membranous localization of α- and β-catenin (arrowheads), and accumulation of ghost cells in the lumen (GC). (F) Stage V: Loss of α-catenin expression, lack of detectable membranous β-catenin expression (arrowheads), and keratin-like deposition in the lumen (KE). Tissue from day 15 of pregnancy (A–E); involuted tissue (F).
Activation of β-Catenin Using MMTV-Cre also Results in Transdifferentiation of Mammary Epithelium.
Although the WAP gene promoter activates Cre in differentiating mammary alveoli, the MMTV-LTR activates Cre already in ductal epithelium (15). To investigate whether the early activation of β-catenin during puberty and pregnancy also led to transdifferentiation into metaplasia, we deleted exon 3 of the β-catenin gene by using MMTV-Cre transgenic mice (Catnb+/Δex3:MMTVcre). These mice, in contrast to the Catnb+/Δex3:WAPcre mice, were unable to lactate even after the first pregnancy. Mammary tissue from virgin 5-mo-old female mice contained scattered loci of squamous metaplasia (not shown), which filled the entire mammary gland after a single pregnancy (Fig. 6 A and B). Extensive infiltration of inflammatory cells but no obvious neoplastic lesions were detected. Expression of K1 and K5 were observed in hyperplastic structures and squamous cells (Fig. 6 C and D), and NKCC1 expression was reduced (Fig. 6E). This phenotype was very similar to that seen in Catnb+/Δex3:WAPcre mice.
Figure 6.
Stabilization of β-catenin through MMTV-Cre-induced deletion of exon 3 causes metaplasia. (A and B) Hematoxylin/eosin stained section of mammary tissue from a 5-mo-old Catnb+/Δex3:MMTVCre mouse after one pregnancy. (C–E) Immunofluorescent staining for K1 (red; C), K5 (red; D), and NKCC1 (red, E). The green color represents β-catenin staining. The phenotype was similar to that observed in Catnb+/Δex3:WAPCre mice. K1 was detected in the inside of nodules, and K5 was detected in the outer and inner layer of the nodules in Catnb+/Δex3:MMTVCre. NKCC1 was partially lost in areas with accumulated β-catenin (arrowhead).
Previous studies have shown that expression of an N-terminally truncated β-catenin (ΔN89 β-catenin) under the control of the MMTV-LTR resulted in precocious alveolar development and the formation of adenocarcinomas in virgin mice (10). Similarly, an N-terminally truncated β-catenin (ΔN90 β-catenin) under the control of the MMTV-LTR caused adenocarcinomas indistinguishable from Wnt-induced tumors (20). The stabilized β-catenin used in our study lacks amino acids 5–80, whereas those of Imbert et al. (10) lacks amino acids 1–89, and that of Michaelson and Leder (20) lacks the N-terminal 90 aa. It is therefore possible that the phenotypic differences are the result of biochemical differences between the two proteins. Alternatively, it is feasible that the cell types targeted and/or the expression levels are the cause for the observed differences. However, the discrepancies (see Fig. 7 for summary) can probably not be explained by the cell type targeted because an MMTV-LTR was used in all cases. It is possible that differences in β-catenin levels in the two studies led to the differences in phenotype. Specifically, the MMTV-ΔN89 and MMTV-ΔN90 β-catenin transgenes active only in hormone-responsive cells. In contrast, the endogenous β-catenin in this study is stabilized in all cell types that are derived from a cell that has undergone Cre-mediated recombination and still expresses the endogenous gene. Mice in which β-catenin had been stabilized through the deletion of exon 3 did not develop adenocarcinomas over a period of 5 mo and several pregnancies. In contrast, 90% of the MMTV-ΔN89 β-catenin transgenic mice had tumors at the age of 5 mo (10) and 70% of the MMTV-ΔN89 mice developed tumors by 15 mo of age (20).
Figure 7.
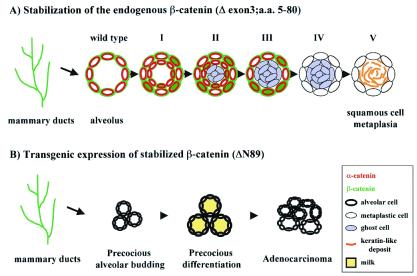
Schematic representation of the differences observed between stabilization of β-catenin through the deletion of exon 3 (loss of amino acids 5–80) in ductal and alveolar epithelium by using MMTV-Cre mice and in alveolar epithelium during pregnancy by using WAP-Cre mice (A), and expression of stabilized β-catenin (loss of amino acids 1–89) by using an MMTV-based transgene (B; ref. 10). (A) Stages of transdifferentiation. Wild-type: Alveoli exhibit basolateral α- and β-catenin staining. Stage I: Local cytoplasmic accumulation of β-catenin. Stage II: Increased local cytoplasmic accumulation of β-catenin, normal membranous localization of α-catenin and the formation of epidermal cysts. Stage III: Accumulation of ghost cells in the lumen of the epidermal cysts accompanied by membranous localization of α- and β-catenin. Stage IV: Complete loss of α- and β-catenin accompanied by increased accumulation of ghost cells in the lumen. Stage V: No definitive α- and β-catenin staining, appearance of keratin-like deposits in the lumen. (B) Stages of differentiation and transformation of mammary epithelium in mice expressing stabilized β-catenin under control of the MMTV-LTR. This diagram is based on published data (10). Precocious alveolar budding occurred during early (4 wk) virgin development. By 12 wk, premature differentiation of alveoli was apparent as assessed by histological analyses and milk protein gene expression. Virgin females (6–8 mo old) presented with adenocarcinomas. α-catenin, red; β-catenin, green; keratin-like deposits, orange.
In summary, we demonstrate that stabilization of β-catenin in differentiating alveolar epithelium results in the transdifferentiation of these cells into epidermal and pilar structures. In contrast to other systems, no adenocarcinomas developed over a time period of 6 mo. Although others (10, 20) have not described the pilar differentiation that we describe in the early lesions in our mice, examination of some of the slides (20) revealed that their tumors have considerable evidence of pilar differentiation (R.D.C., unpublished data). Finally, we propose that suppression of β-catenin signaling in the developing and differentiating mammary gland is fundamental for the maintenance of mammary secretory cell fate and identity.
Acknowledgments
We acknowledge Drs. Gertraud W. Robinson, Katherine Walton, and Ryugo Okagaki for critically reviewing the manuscript and Priscilla Furth for introducing us to the metaplastic transformations. K.M. thanks Professor Hideo Inoue for his encouragement. M.M.T. is supported by grants from Ministry of Education, Science, Sports and Culture; and Organization for Pharmaceutical Safety and Research, Japan. Part of this work was supported by a grant from the Director of the National Cancer Institute (to L.H.). K.K. is grateful to Dr. Paul A. W. Edwards for his interest and encouragement, and acknowledges support from National Colorectal Research Alliance, Dana–Farber Cancer Institute.
Abbreviations
- LEF
lymphoid enhancer transcription factor
- MMTV-LTR
mouse mammary tumor virus-long terminal repeat
- WAP
whey acidic protein
- NKCC1
Na-K-Cl cotransporter 1
- Npt2b
Na-Pi cotransporter type II b
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
L.H. and K.K. contributed equally to this work.
References
- 1.McCrea P D, Turck C W, Gumbiner B. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- 2.Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, Logtenberg T, Clevers H. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- 3.Hovanes K, Li T W, Munguia J E, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe R F, Waterman M L. Nat Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 4.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Development (Cambridge, UK) 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 5.Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widelitz R B, Jiang T X, Lu J, Chuong C M. Dev Biol. 2000;219:98–114. doi: 10.1006/dbio.1999.9580. [DOI] [PubMed] [Google Scholar]
- 7.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 8.van Genderen C, Okamura R M, Farinas I, Quo R G, Parslow T G, Bruhn L, Grosschedl R. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Hively W P, Varmus H E. Oncogene. 2000;19:1002–1009. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- 10.Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P. J Cell Biol. 2001;153:555–568. doi: 10.1083/jcb.153.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo M M. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner K U, Wall R J, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth P A, Hennighausen L. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pittius C W, Sankaran L, Topper Y J, Hennighausen L. Mol Endocrinol. 1988;2:1027–1032. doi: 10.1210/mend-2-11-1027. [DOI] [PubMed] [Google Scholar]
- 14.Robinson G W, McKnight R A, Smith G H, Hennighausen L. Development (Cambridge, UK) 1995;121:2079–2090. doi: 10.1242/dev.121.7.2079. [DOI] [PubMed] [Google Scholar]
- 15.Wagner K-U, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L. Transgenic Res. 2001;10:545–553. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]
- 16.Walton K D, Wagner K-U, Rucker E B, III, Shillingford J M, Miyoshi K, Hennighausen L. Mech Dev. 2001;109:281–293. doi: 10.1016/s0925-4773(01)00549-4. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Robinson G W, Hennighausen L. Mol Endocrinol. 1996;10:1496–1506. doi: 10.1210/mend.10.12.8961260. [DOI] [PubMed] [Google Scholar]
- 18.Onuma H, Mastui C, Morohashi M. Arch Dermatol Res. 2001;293:133–138. doi: 10.1007/s004030000195. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs E, Byrne C. Curr Opin Genet Dev. 1994;4:725–736. doi: 10.1016/0959-437x(94)90140-x. [DOI] [PubMed] [Google Scholar]
- 20.Michaelson J S, Leder P. Oncogene. 2001;20:5093–5099. doi: 10.1038/sj.onc.1204586. [DOI] [PubMed] [Google Scholar]