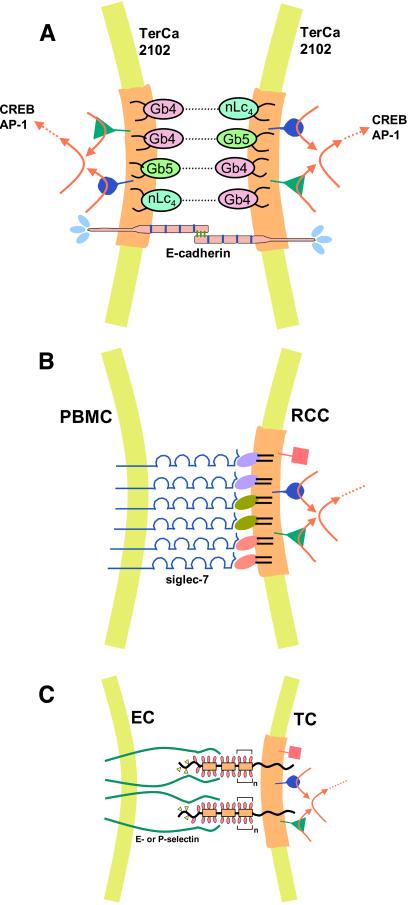
Figure 3.
Models of glycosylation-dependent cell adhesion with signaling. (A) Self-adhesion (autoaggregation) of human teratocarcinoma 2102 cells, with simultaneous signaling to activate transcription factors. The adhesion is mediated by two globo-series (Gb4, Gb5) and one lacto-series GSL (nLc4) based on Gb4–nLc4 or Gb4–Gb5 interaction in type 1 glycosynapse. Structures: Gb4, GalNAcβ3Galα4Galβ4Glcβ1Cer; nLc4, Galβ4GlcNAcβ3Galβ4Glcβ1Cer; Gb5, see Table 1. Adhesion by this process may occur in cooperation with E-cadherin-based homotypic interaction. The former process may be much faster than the latter. The glycosynapse membrane is indicated by a light brown color. Simultaneous with GSL-dependent adhesion, signal transducers (cSrc, RhoA, RasH) present in this glycosynapse may be activated, leading to activation of transcription factors AP-1 (activation protein 1) or CREB (cAMP responsive element binding protein). The adhesion process can be inhibited by Abs directed to Gb4, Gb5, or nLc4. (B) Adhesion of renal cell carcinoma (RCC) to peripheral blood mononuclear cells (PBMC), mediated by clustered disialogangliosides in type 1 glycosynapse, and binding of siglec-7 expressed at the PBMC surface. Three types of disialoganglioside in RCC, indicated by different colors, are organized with signal transducers (cSrc, RhoA, focal adhesion kinase) present in RCC glycosynapse. Disialogangliosides of RCC that bind to siglec-7 are GalNAc-disialyl-Lc4, disialyl-Lc4, and disialyl-Gb5 (see Table 1 for structures). Siglec-7 binds equally well to the three types of disialoganglioside, indicating a lack of binding specificity. Low binding specificity or lack of binding specificity in endogenous lectin is often seen in selectins, siglecs, and galectins. Adhesion of RCC to PBMC causes large-scale aggregation of these two types of cells, which may lead to microembolisms in lung. RCC adhesion to PBMC activates cSrc, followed by signaling (red arrows) to enhance motility and invasiveness. (C) Tumor cell (TC) adhesion to activated ECs, through type 2 glycosynapse in TCs, may activate transducers, followed by signaling to enhance TC motility and invasiveness. Mucin-type transmembrane glycoproteins are organized with signal transducers in glycosynapse (light brown). The majority of glycosyl epitopes in TCs involved in selectin-dependent adhesion are carried by mucin-type glycoproteins in type 2 glycosynapse. The glycosyl epitopes are SLex, SLex-Lex, and SLea, which bind to E-selectin but not to P-selectin unless they are expressed on PSGL-1, and sulfated SLex, which binds to P-selectin. This process, i.e., binding of activated ECs to type 2 glycosynapse of TCs, may activate Src family kinases, RhoA, and Ras present in the glycosynapse, leading to enhanced motility and invasiveness.