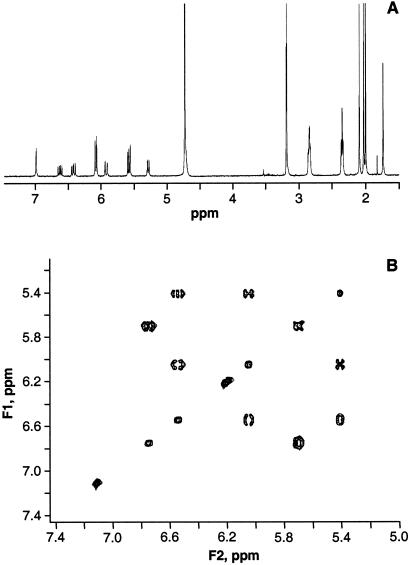
Figure 7.
NMR one-dimensional 1H spectrum and total correlation spectroscopy (TOCSY) spectra of the 23.3-min HPLC fraction identified it as biliverdin IXα. (A) The one-dimensional 1H spectrum of the pure 23.3-min HPLC fraction is identical to that of commercial biliverdin IXα. Chemical shifts are relative to trimethylsilyl propionate at 0.00 ppm: 1H NMR (methanol-d4) δ 6.54(m, 1H, H-21), 5.41(d, 1H, H-22), 6.05(d, 1H, H-22′), 6.22(s, 1H, H-5), 7.11(s, 1H, H-10), 6.19(s, 1H, H-15), 6.75(m, 1H, H-171), 5.72(d, 1H, H-172), 5.68(m, 1H, H-172′), 2.47(t, 4H, H-82, H-122), 2.96(t, 4H, H-81, H-121), 2.09, 2.02, 2.00, 1.73(s, 12H, H-31, H-71, H-131, H-181); 13C NMR (methanol-d4) δ 173.3(C-1, C-19), 127.1(C-21), 119.9(C-22), 100.0(C-5, C-15), 117.1(C-10), 127.6(C-171), 123.1(C-172), 21.5(C-81, C-121), 38.2(C-82, C-122) 177.8(C-83, C-123), 139.5(C-8, C-12). (B) Plot of the vinyl region from TOCSY spectrum of the oocyte molecule. The chemical shifts and coupling patterns are identical to commercial biliverdin IXα. Additionally, the coupling between the carbonyl carbon and the α and β methylene protons of the propionic acid side chains were verified from the distortionless enhancement by polarization transfer-heteronuclear multiple quantum correlation and heteronuclear multiple bond correlation spectra (data not shown).