Abstract
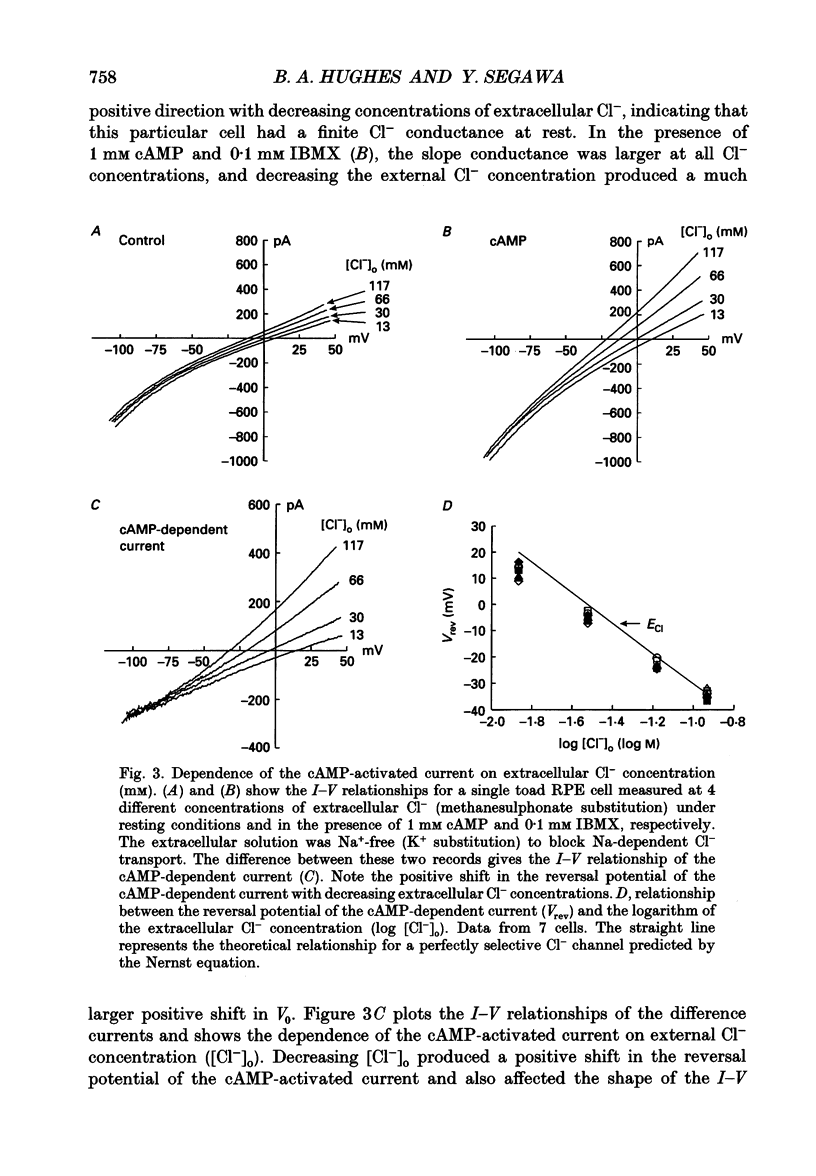
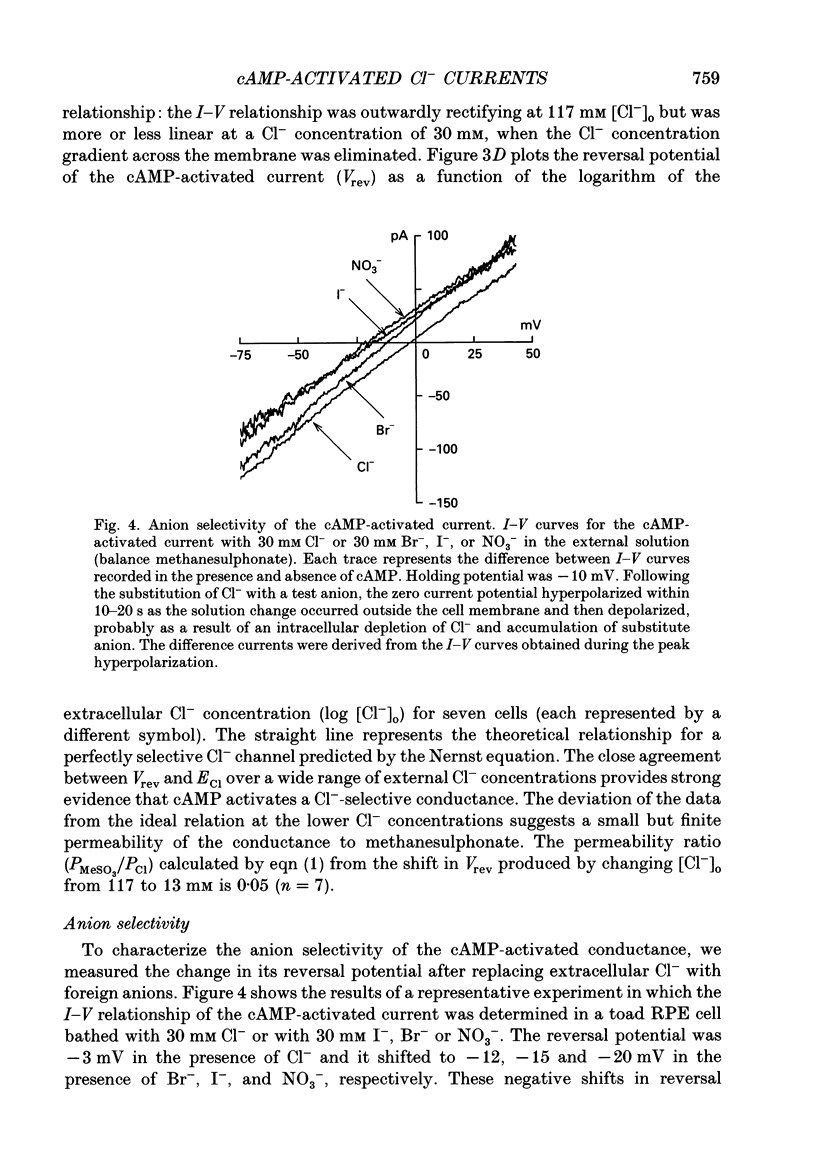
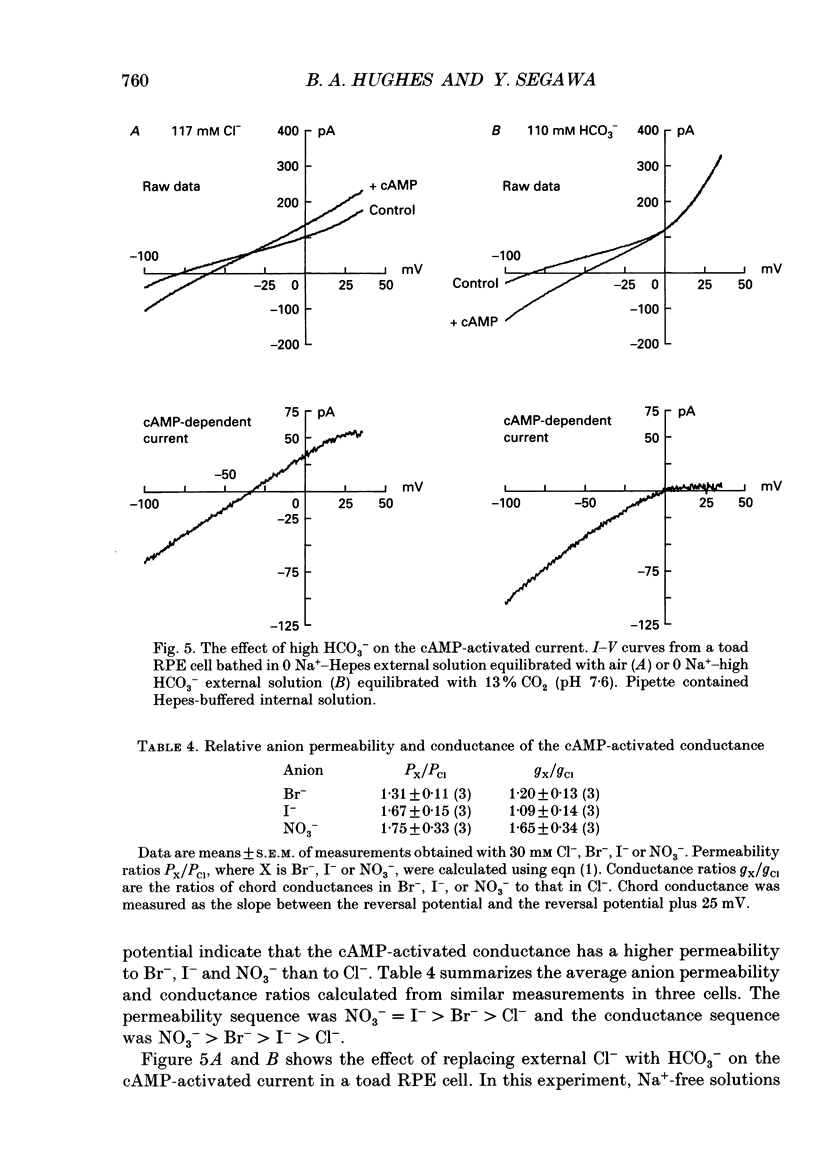
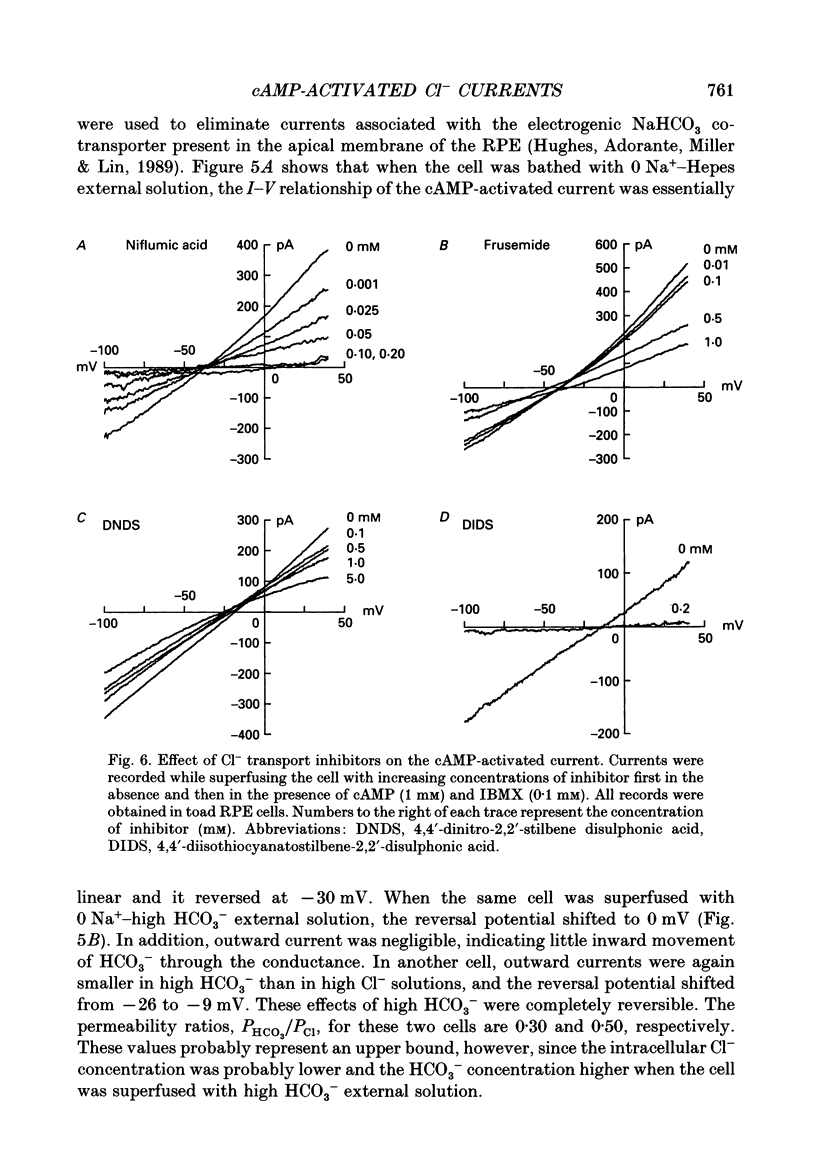
1. The effect of cAMP on whole-cell currents in isolated retinal pigment epithelial (RPE) cells of the bullfrog and marine toad was investigated by means of the perforated patch clamp technique. 2. Superfusing cells with either cAMP or forskolin led to the development of a time-independent current that had a linear current-voltage (I-V) relationship. The reversal potential of (Vrev) of the cAMP-activated current was unaffected by the removal of either Na+ or HCO3- from the external and internal solutions or by the addition of extracellular barium, but it was near the Cl- equilibrium potential (ECl) over a wide range of extracellular Cl- concentrations, suggesting the presence of a Cl(-)-selective channel. 3. The anion permeability sequence of the cAMP-activated conductance calculated from biionic reversal potentials was NO3- = I- > Br- > Cl- >> HCO3- > methanesulphonate. 4. The conductance was blocked by a variety of Cl- transport inhibitors, including 4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid (DIDS), 4,4'-dinitro-2,2'- stilbene disulphonic acid (DNDS), frusemide, N-phenylanthranilic acid (DPC) and niflumic acid. 5. The present study demonstrates that cAMP activates a Cl(-)-selective channel that most probably resides in the basolateral membrane.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorante J. S., Miller S. S. Potassium-dependent volume regulation in retinal pigment epithelium is mediated by Na,K,Cl cotransport. J Gen Physiol. 1990 Dec;96(6):1153–1176. doi: 10.1085/jgp.96.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Gallemore R. P., Hughes B. A., Steinberg R. H. Direct evidence for a basolateral membrane Cl- conductance in toad retinal pigment epithelium. Am J Physiol. 1992 Feb;262(2 Pt 1):C374–C383. doi: 10.1152/ajpcell.1992.262.2.C374. [DOI] [PubMed] [Google Scholar]
- Fuller C. M., Benos D. J. CFTR! Am J Physiol. 1992 Aug;263(2 Pt 1):C267–C286. doi: 10.1152/ajpcell.1992.263.2.C267. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Argent B. E. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988 Oct;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. A., Adorante J. S., Miller S. S., Lin H. Apical electrogenic NaHCO3 cotransport. A mechanism for HCO3 absorption across the retinal pigment epithelium. J Gen Physiol. 1989 Jul;94(1):125–150. doi: 10.1085/jgp.94.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. A., Miller S. S., Farber D. B. Adenylate cyclase stimulation alters transport in frog retinal pigment epithelium. Am J Physiol. 1987 Apr;252(4 Pt 1):C385–C395. doi: 10.1152/ajpcell.1987.252.4.C385. [DOI] [PubMed] [Google Scholar]
- Hughes B. A., Miller S. S., Joseph D. P., Edelman J. L. cAMP stimulates the Na+-K+ pump in frog retinal pigment epithelium. Am J Physiol. 1988 Jan;254(1 Pt 1):C84–C98. doi: 10.1152/ajpcell.1988.254.1.C84. [DOI] [PubMed] [Google Scholar]
- Hughes B. A., Miller S. S., Machen T. E. Effects of cyclic AMP on fluid absorption and ion transport across frog retinal pigment epithelium. Measurements in the open-circuit state. J Gen Physiol. 1984 Jun;83(6):875–899. doi: 10.1085/jgp.83.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
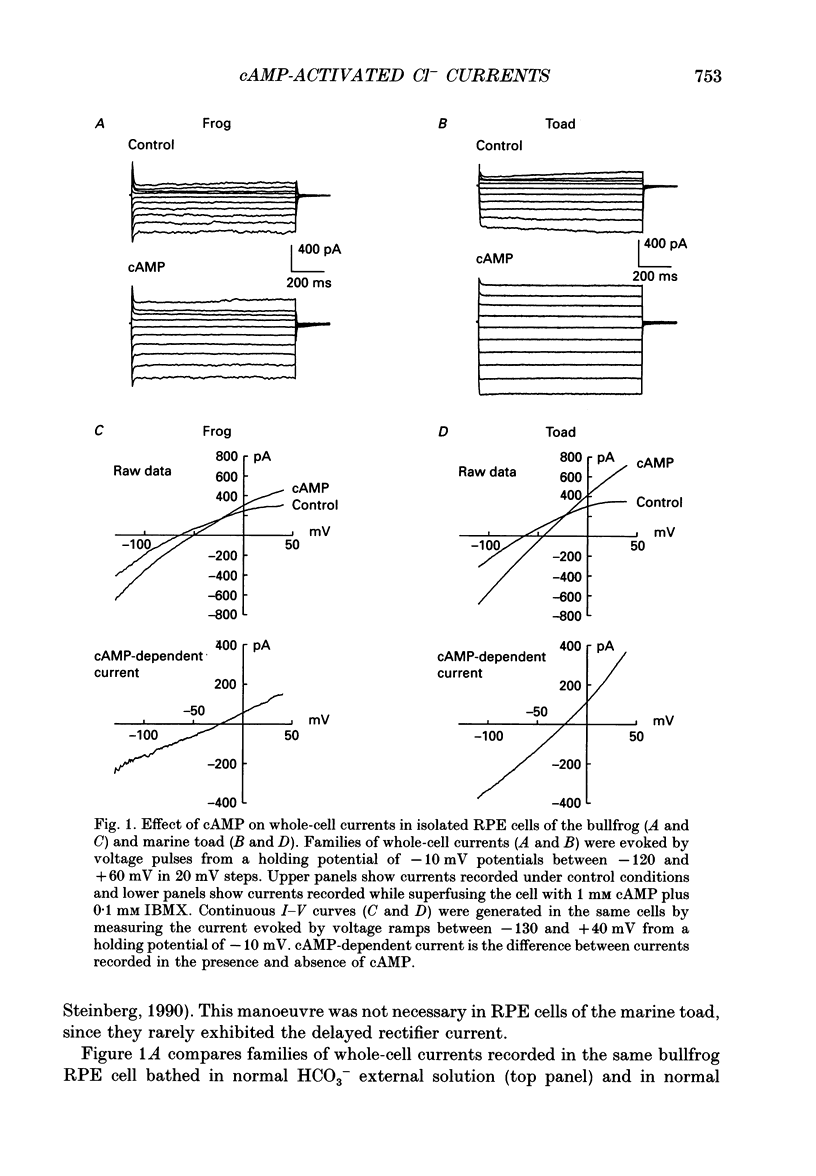
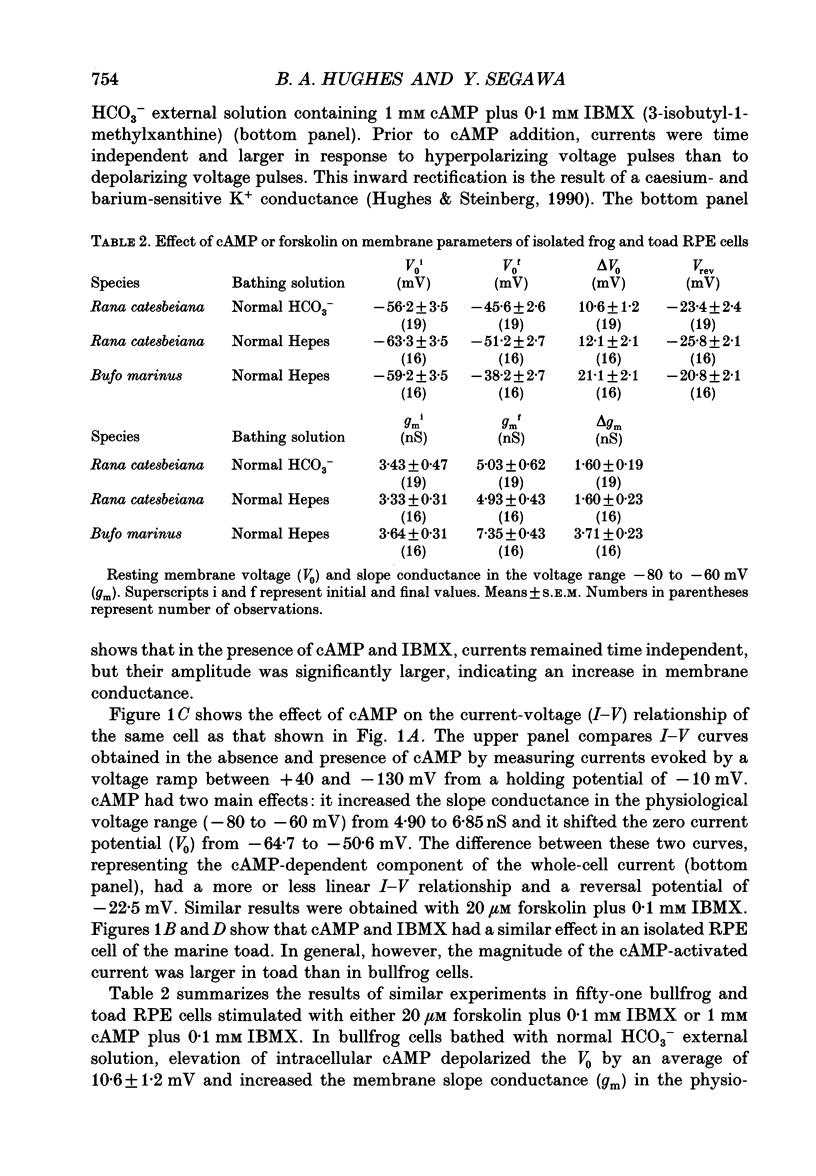
- Hughes B. A., Steinberg R. H. Voltage-dependent currents in isolated cells of the frog retinal pigment epithelium. J Physiol. 1990 Sep;428:273–297. doi: 10.1113/jphysiol.1990.sp018212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D. P., Miller S. S. Apical and basal membrane ion transport mechanisms in bovine retinal pigment epithelium. J Physiol. 1991 Apr;435:439–463. doi: 10.1113/jphysiol.1991.sp018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Cour M. Cl- transport in frog retinal pigment epithelium. Exp Eye Res. 1992 Jun;54(6):921–931. doi: 10.1016/0014-4835(92)90156-m. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Edelman J. L. Active ion transport pathways in the bovine retinal pigment epithelium. J Physiol. 1990 May;424:283–300. doi: 10.1113/jphysiol.1990.sp018067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. S., Steinberg R. H. Passive ionic properties of frog retinal pigment epithelium. J Membr Biol. 1977 Sep 15;36(4):337–372. doi: 10.1007/BF01868158. [DOI] [PubMed] [Google Scholar]
- Miller S., Farber D. Cyclic AMP modulation of ion transport across frog retinal pigment epithelium. Measurements in the short-circuit state. J Gen Physiol. 1984 Jun;83(6):853–874. doi: 10.1085/jgp.83.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Lavie J. L., Mironneau C., Mironneau J. Calcium-activated chloride current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1989 Apr;413(6):629–636. doi: 10.1007/BF00581813. [DOI] [PubMed] [Google Scholar]
- Rae J. L., Cooper K. New techniques for the study of lens electrophysiology. Exp Eye Res. 1990 Jun;50(6):603–614. doi: 10.1016/0014-4835(90)90101-y. [DOI] [PubMed] [Google Scholar]
- Rao M. C., Nash N. T., Field M. Differing effects of cGMP and cAMP on ion transport across flounder intestine. Am J Physiol. 1984 Jan;246(1 Pt 1):C167–C171. doi: 10.1152/ajpcell.1984.246.1.C167. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- Wiederholt M., Zadunaisky J. A. Decrease of intracellular chloride activity by furosemide in frog retinal pigment epithelium. Curr Eye Res. 1984 Apr;3(4):673–675. doi: 10.3109/02713688409003071. [DOI] [PubMed] [Google Scholar]


