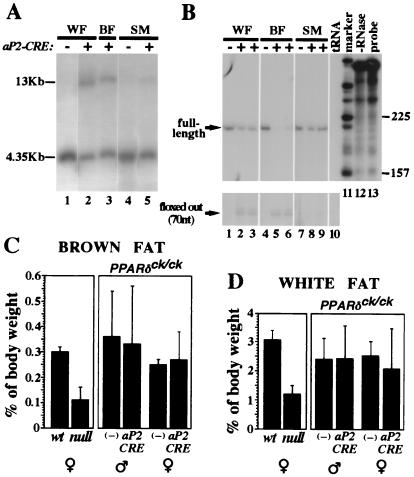
Figure 4.
Adipocyte-specific PPARδ knockout does not affect adipose tissue mass. (A) Southern blot analysis of epidydimal white fat pad (WF), interscapular brown fat pad (BF), and thigh skeletal muscle (SM) from mice carrying the PPARδck/ck allele and an aP2-CRE transgene. A 4.35-kb EcoRI fragment represents the nonexcised PPARδck allele, whereas CRE-mediated recombination yields a 13-kb EcoRI fragment (see Fig. 1 and Materials and Methods for further detail). Notice that CRE-dependent conversion into the 13-kb band is substantial in white and brown fat (lanes 2 and 3), and residual in skeletal muscle (lane 5). (B) RNase protection analysis of PPARδ transcript structure in the same mice. CRE-mediated deletion modifies a 210-nt-long protected fragment representing the full-length transcript (see arrow) into a 70-nt-long fragment representing a functionally null transcript devoid of its fourth exon (arrow, Bottom). The assay reveals ≈50% aP2CRE-mediated deletion of the full-length PPARδ mRNA in white fat (lanes 2 and 3 vs. lane 1), >80% in brown fat (lanes 5 and 6 vs. lane 4) and only a marginal one in skeletal muscle (lanes 8 and 9 vs. lane 7). Specificity controls with yeast tRNA and no RNase are shown in lanes 10 and 12, respectively. (C and D) Relative weights of interscapular brown fat pads (C) and epidydimal white fat pads (D) in wt and PPARδ null mice, and in PPARδck/ck mice in the absence (−) or presence of the aP2-CRE transgene. The 2.5- to 3-fold differences in adipose mass between wt and null mice (Left) are not recapitulated by an adipose-specific gene knockout (aP2-CRE vs. −, Right).