Abstract
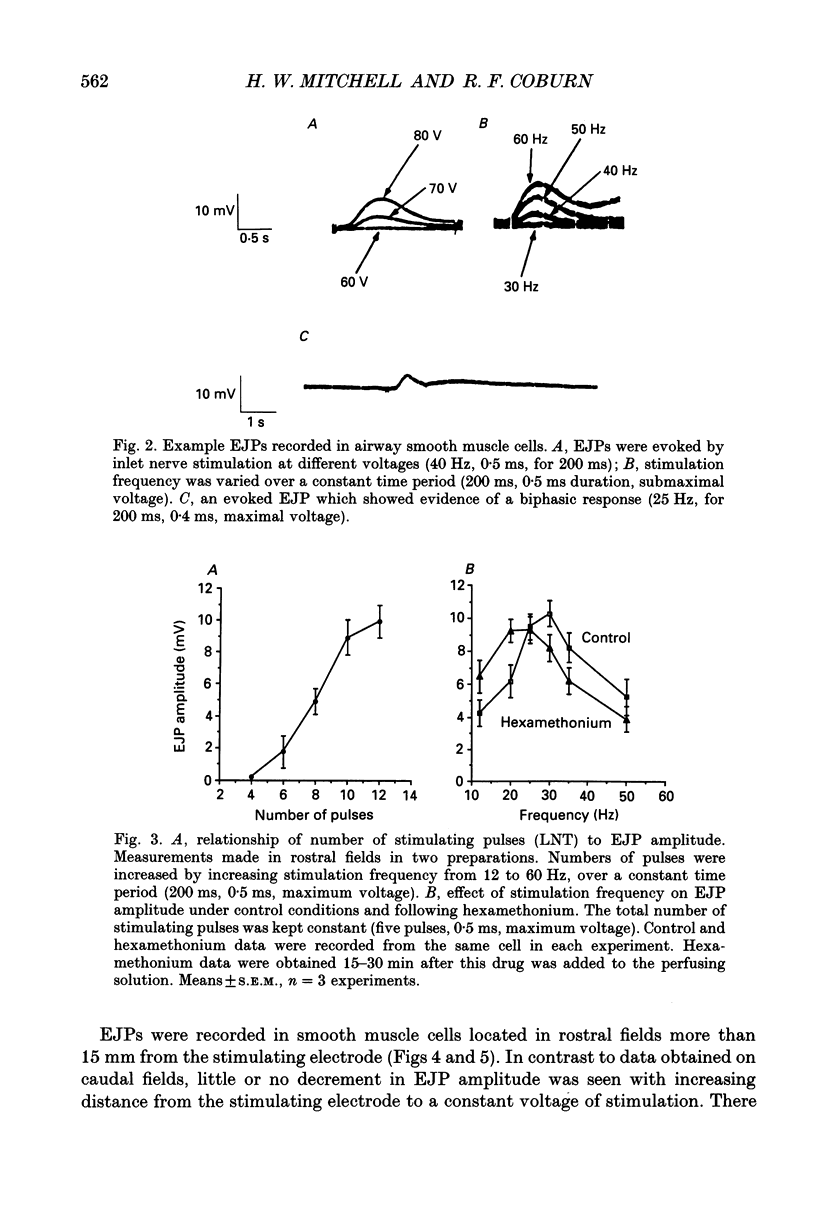
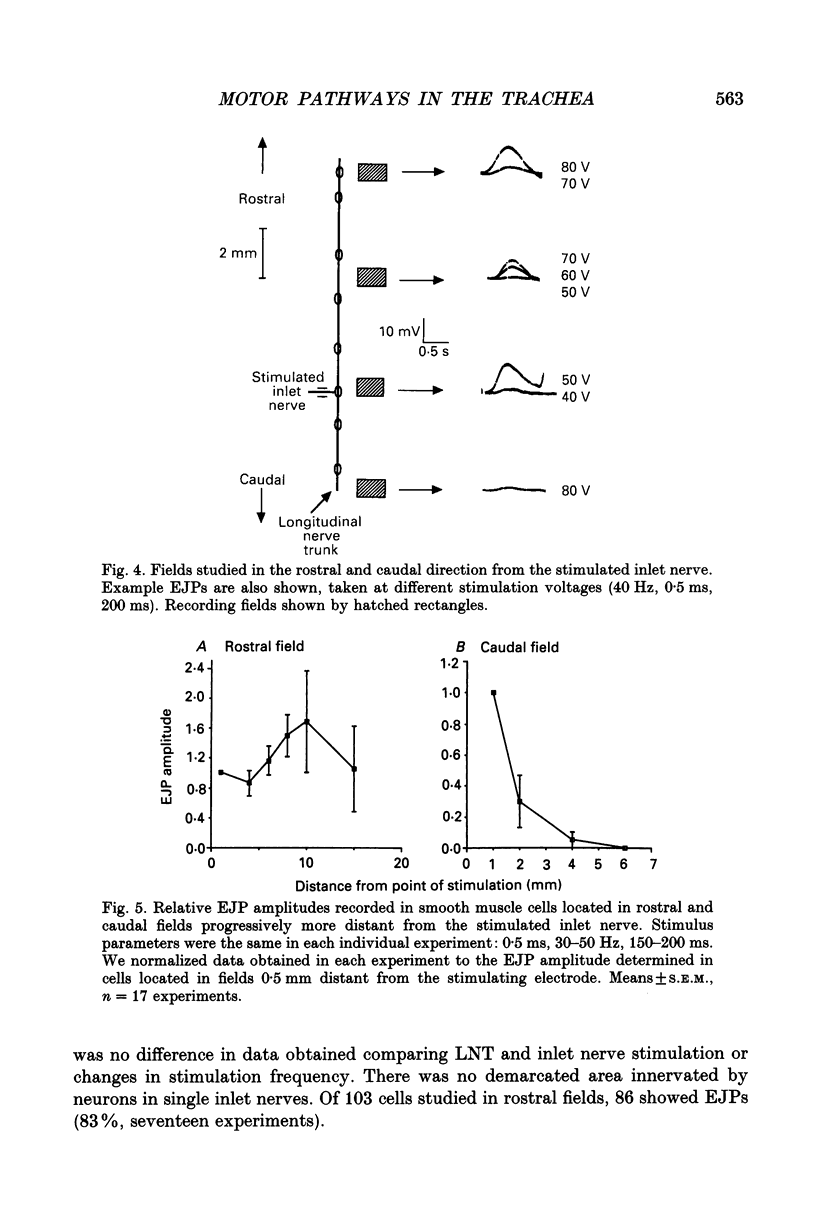
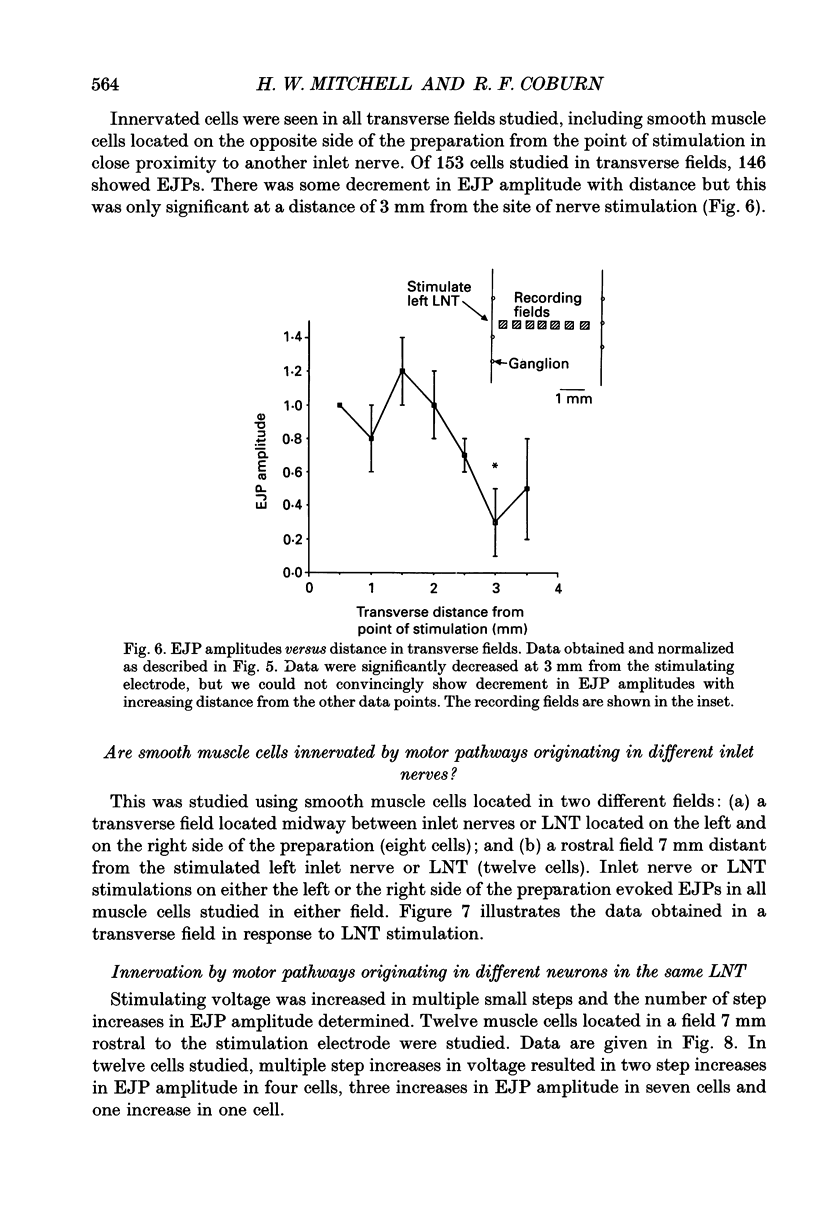
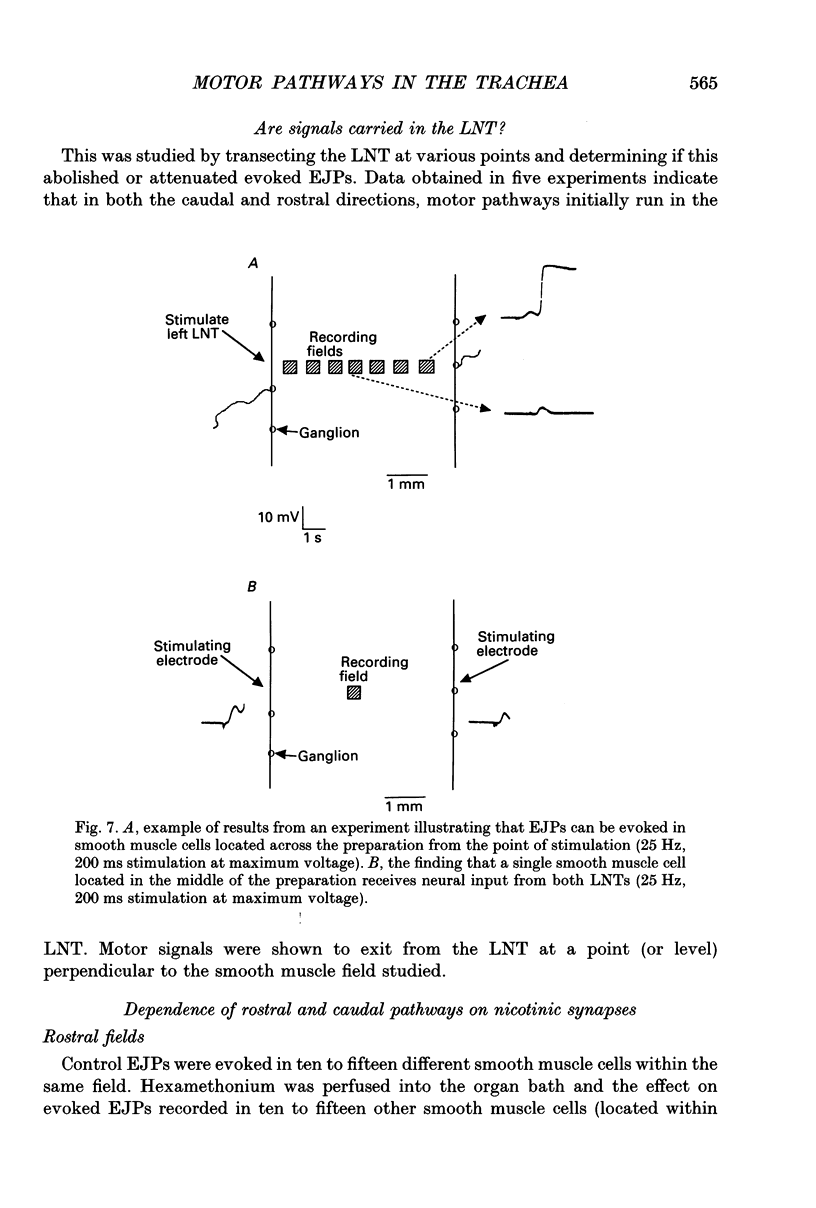
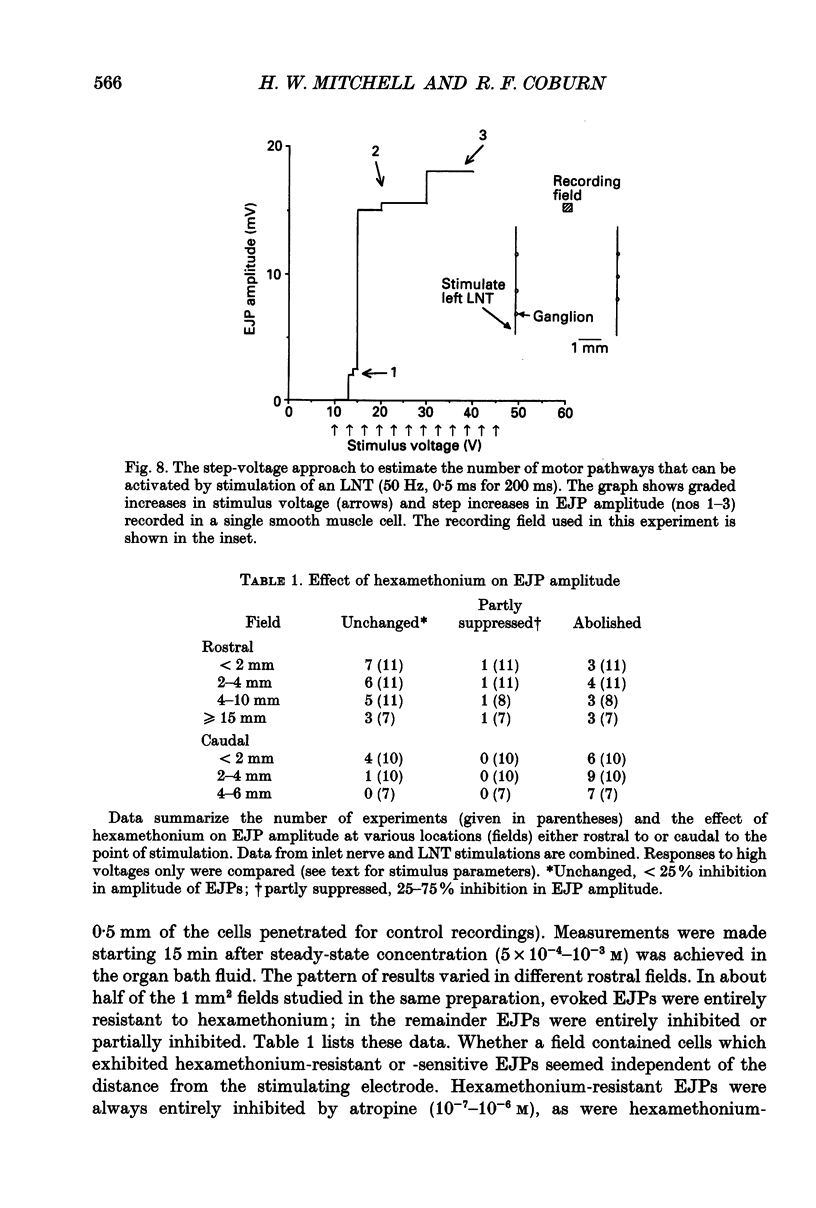
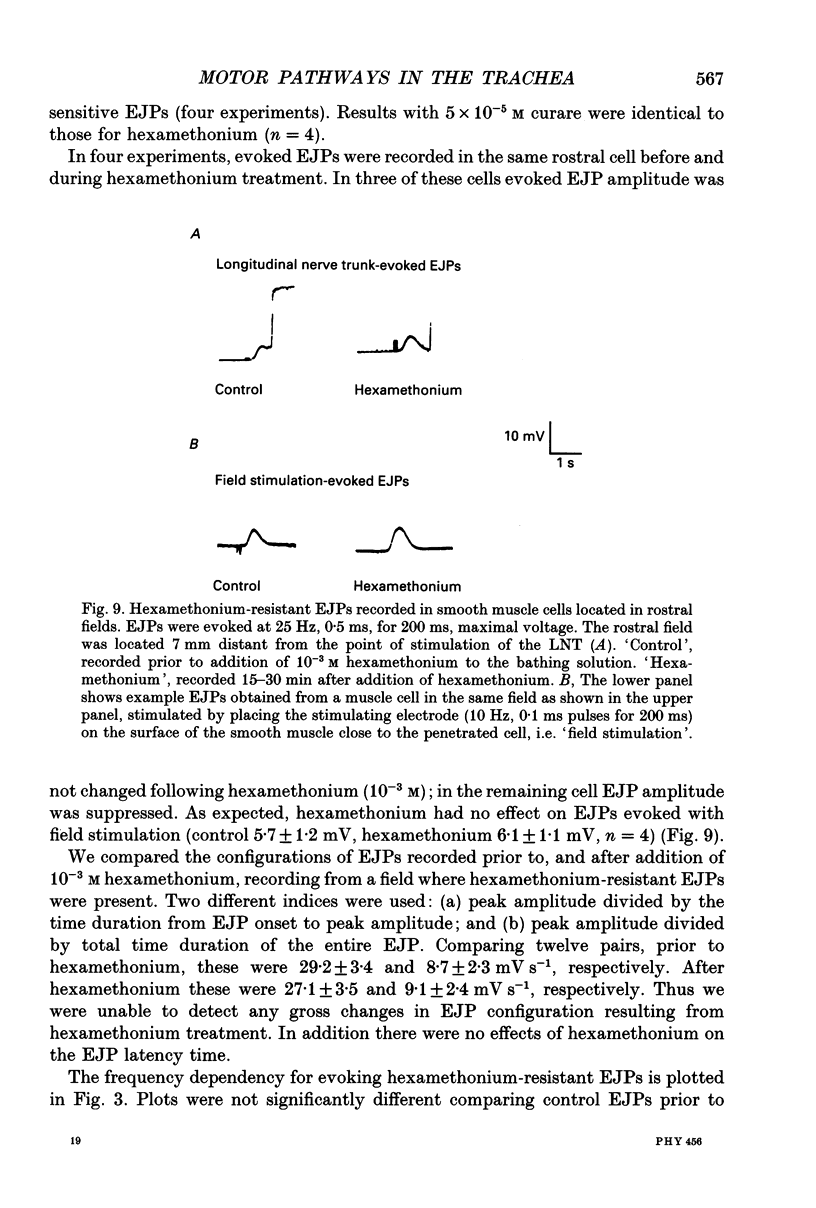
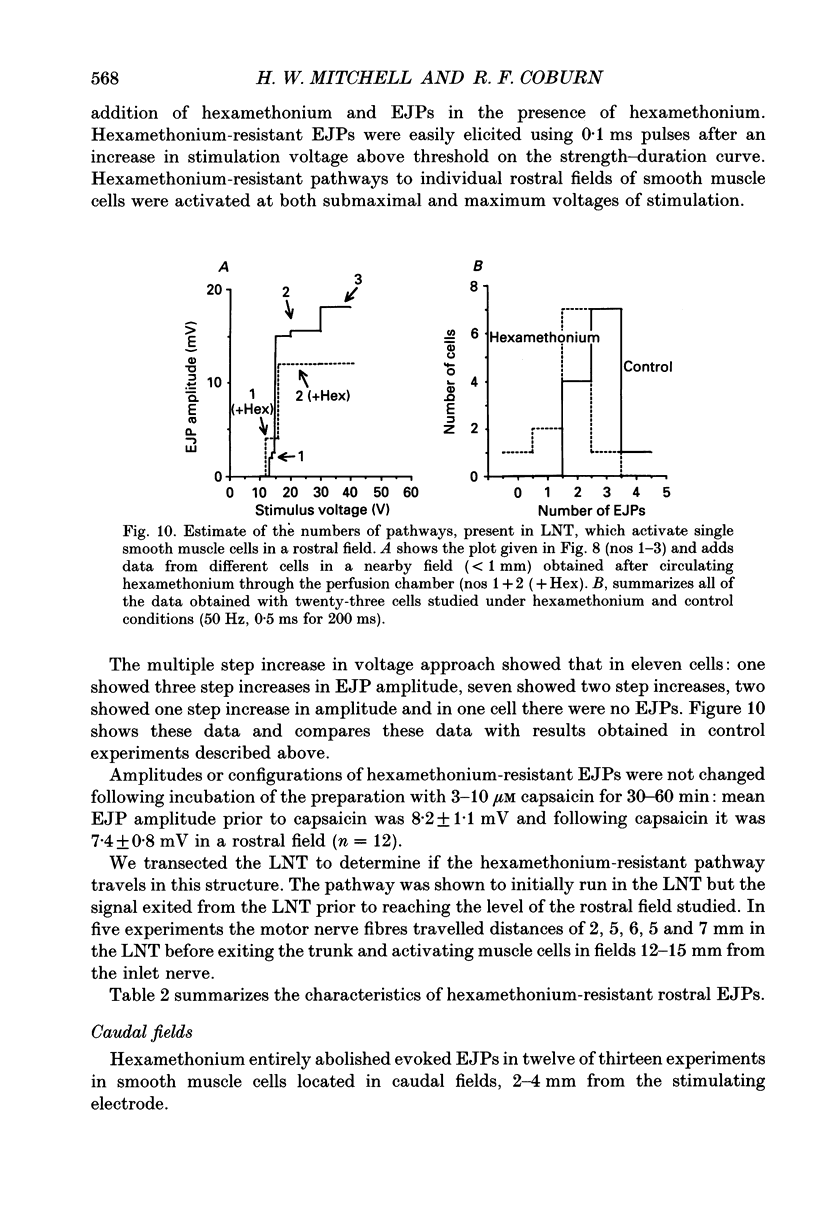
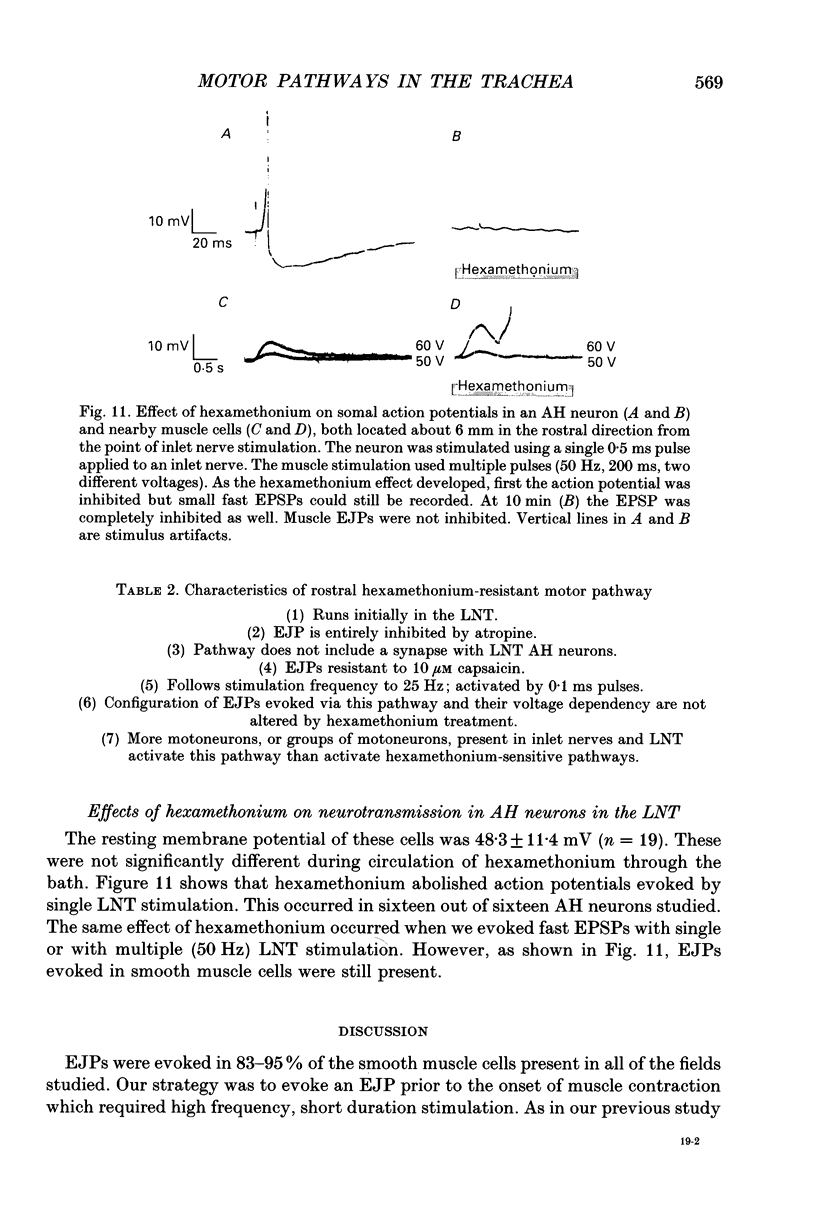
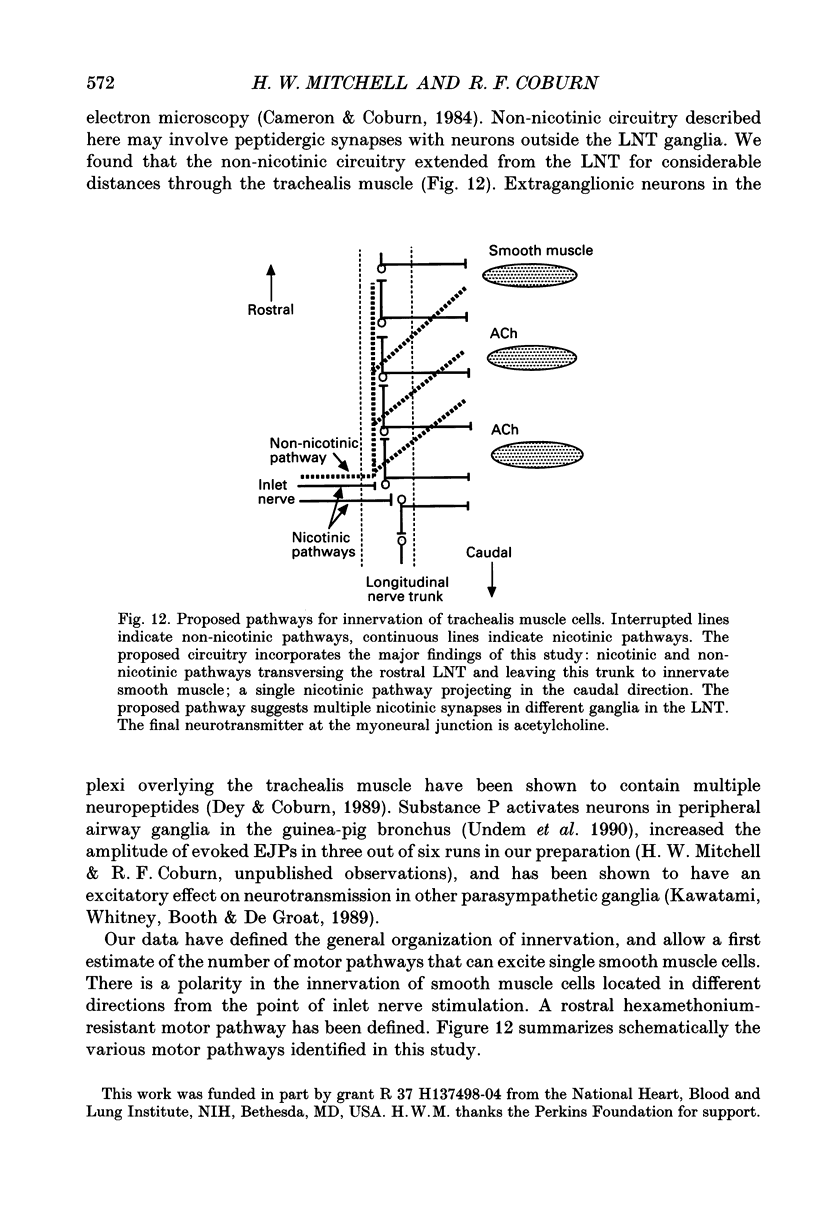
1. We investigated the distribution and characteristics of motor pathways to individual smooth muscle cells activated by electrical stimulation of either, single nerves which enter the tracheal plexus (inlet nerves), or a longitudinal nerve trunk (LNT) located near the entrance of an inlet nerve into the plexus. Excitatory junction potentials (EJPs) were recorded using intracellular microelectrodes as an index of smooth muscle cell activation. In all experiments EJPs were completely blocked by tetrodotoxin and by atropine. 2. In smooth muscle fields located in the caudal direction from the point of inlet or LNT nerve stimulation, neural input decreased as a function of distance. There was evidence of a demarcated area innervated by neurons entering the plexus in one inlet nerve. In smooth muscle fields located in the rostral or transverse direction from the site of nerve stimulation, no such demarcated area could be identified. 3. Of the smooth muscle cells located within the innervated fields studied, 83-95% were activated following stimulation of a single inlet nerve or LNT. Evoked EJPs were similar in different innervated cells or units of electrically coupled cells located within the same 1 mm2 'field'. 4. There was overlapping cholinergic motor input to single smooth muscle cells originating from neurons present in different inlet nerves or different neurons present in the same inlet nerve or region of the LNT. Multiple small step increases in the voltage used to stimulate a LNT resulted in three or four step increases in EJP amplitudes. This gives a minimal value for the number of motor pathways that can be activated by neurons in a region of LNT leading to a single smooth muscle cell. 5. Motor pathways to smooth muscle cells located in caudal and rostral fields ran initially in the LNT and exited in proximity to the smooth muscle cell studied. 6. Motor pathways used in transmitting signals to smooth muscle cells to different areas of trachealis muscle varied in their sensitivity to hexamethonium or curare. EJPs evoked in fields located in the caudal direction from the stimulating electrode were abolished by these drugs. Muscle cells located in different rostral fields showed EJPs that were either sensitive or resistant to these drugs. 7. The rostral hexamethonium-resistant pathway ran initially in the LNT but it exited from the LNT several millimetres before reaching the level of the smooth muscle field innervated. This pathway followed stimulation frequencies up to 25 Hz. The final neuron in this pathway released acetylcholine and evoked EJPs were entirely inhibited by atropine.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. G., Burnstock G. A voltage-clamp study of the electrophysiological characteristics of the intramural neurones of the rat trachea. J Physiol. 1990 Apr;423:593–614. doi: 10.1113/jphysiol.1990.sp018042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. G., Basbaum C. B., Herbert D. A., Mitchell R. A. Transmission in airway ganglia of ferrets: inhibition by norepinephrine. Neurosci Lett. 1983 Oct 31;41(1-2):139–143. doi: 10.1016/0304-3940(83)90236-7. [DOI] [PubMed] [Google Scholar]
- Baker D. G., McDonald D. M., Basbaum C. B., Mitchell R. A. The architecture of nerves and ganglia of the ferret trachea as revealed by acetylcholinesterase histochemistry. J Comp Neurol. 1986 Apr 22;246(4):513–526. doi: 10.1002/cne.902460408. [DOI] [PubMed] [Google Scholar]
- Bałuk P., Gabella G. Innervation of the guinea pig trachea: a quantitative morphological study of intrinsic neurons and extrinsic nerves. J Comp Neurol. 1989 Jul 1;285(1):117–132. doi: 10.1002/cne.902850110. [DOI] [PubMed] [Google Scholar]
- Blackman J. G., McCaig D. J. Studies on an isolated innervated preparation of guinea-pig trachea. Br J Pharmacol. 1983 Dec;80(4):703–710. doi: 10.1111/j.1476-5381.1983.tb10061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borson D. B. Roles of neutral endopeptidase in airways. Am J Physiol. 1991 Apr;260(4 Pt 1):L212–L225. doi: 10.1152/ajplung.1991.260.4.L212. [DOI] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Electrical activity at the sympathetic neuroeffector junction in the guinea-pig vas deferens. J Physiol. 1988 May;399:607–632. doi: 10.1113/jphysiol.1988.sp017099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas G. A., Graf P. D., Nadel J. A. Sympathetic versus parasympathetic nervous regulation of airways in dogs. J Appl Physiol. 1971 Nov;31(5):651–655. doi: 10.1152/jappl.1971.31.5.651. [DOI] [PubMed] [Google Scholar]
- Cameron A. R., Coburn R. F. Electrical and anatomic characteristics of cells of ferret paratracheal ganglion. Am J Physiol. 1984 May;246(5 Pt 1):C450–C458. doi: 10.1152/ajpcell.1984.246.5.C450. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Kalia M. P. Morphological features of spiking and nonspiking cells in the paratracheal ganglion of the ferret. J Comp Neurol. 1986 Dec 15;254(3):341–351. doi: 10.1002/cne.902540307. [DOI] [PubMed] [Google Scholar]
- Coburn R. F. Neural coordination of excitation of ferret trachealis muscle. Am J Physiol. 1984 May;246(5 Pt 1):C459–C466. doi: 10.1152/ajpcell.1984.246.5.C459. [DOI] [PubMed] [Google Scholar]
- De Jongste J. C., Mons H., Bonta I. L., Kerrebijn K. F. Nonneural components in the response of fresh human airways to electric field stimulation. J Appl Physiol (1985) 1987 Oct;63(4):1558–1566. doi: 10.1152/jappl.1987.63.4.1558. [DOI] [PubMed] [Google Scholar]
- Holzer P., Gamse R., Lembeck F. Distribution of substance P in the rat gastrointestinal tract--lack of effect of capsaicin pretreatment. Eur J Pharmacol. 1980 Feb 8;61(3):303–307. doi: 10.1016/0014-2999(80)90132-6. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Purves D. Apportionment of the terminals from single preganglionic axons to target neurones in the rabbit ciliary ganglion. J Physiol. 1983 May;338:259–275. doi: 10.1113/jphysiol.1983.sp014672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawatani M., Whitney T., Booth A. M., de Groat W. C. Excitatory effect of substance P in parasympathetic ganglia of cat urinary bladder. Am J Physiol. 1989 Dec;257(6 Pt 2):R1450–R1456. doi: 10.1152/ajpregu.1989.257.6.R1450. [DOI] [PubMed] [Google Scholar]
- Leff A. R., Munoz N. M., Tallet J., David A. C., Cavigelli M. A., Garrity E. R. Autonomic response characteristics of porcine airway smooth muscle in vivo. J Appl Physiol (1985) 1985 Apr;58(4):1176–1188. doi: 10.1152/jappl.1985.58.4.1176. [DOI] [PubMed] [Google Scholar]
- Lichtman J. W. On the predominantly single innervation of submandibular ganglion cells in the rat. J Physiol. 1980 May;302:121–130. doi: 10.1113/jphysiol.1980.sp013233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Brodin E., Saria A. Effects and distribution of vagal capsaicin-sensitive substance P neurons with special reference to the trachea and lungs. Acta Physiol Scand. 1983 Nov;119(3):243–252. doi: 10.1111/j.1748-1716.1983.tb07334.x. [DOI] [PubMed] [Google Scholar]
- McWilliam P. N., Gray S. J. The innervation of tracheal smooth muscle in the ferret. J Auton Nerv Syst. 1990 Jul;30(3):233–238. doi: 10.1016/0165-1838(90)90254-g. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A., Baker D. G., Basbaum C. B. In vivo activity of tracheal parasympathetic ganglion cells innervating tracheal smooth muscle. Brain Res. 1987 Dec 22;437(1):157–160. doi: 10.1016/0006-8993(87)91537-x. [DOI] [PubMed] [Google Scholar]
- Sekizawa K., Tamaoki J., Nadel J. A., Borson D. B. Enkephalinase inhibitor potentiates substance P- and electrically induced contraction in ferret trachea. J Appl Physiol (1985) 1987 Oct;63(4):1401–1405. doi: 10.1152/jappl.1987.63.4.1401. [DOI] [PubMed] [Google Scholar]
- Undem B. J., Myers A. C., Barthlow H., Weinreich D. Vagal innervation of guinea pig bronchial smooth muscle. J Appl Physiol (1985) 1990 Oct;69(4):1336–1346. doi: 10.1152/jappl.1990.69.4.1336. [DOI] [PubMed] [Google Scholar]
- Woolcock A. J., Macklem P. T., Hogg J. C., Wilson N. J., Nadel J. A., Frank N. R., Brain J. Effect of vagal stimulation on central and peripheral airways in dogs. J Appl Physiol. 1969 Jun;26(6):806–813. doi: 10.1152/jappl.1969.26.6.806. [DOI] [PubMed] [Google Scholar]


