Abstract
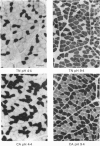
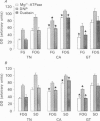
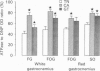
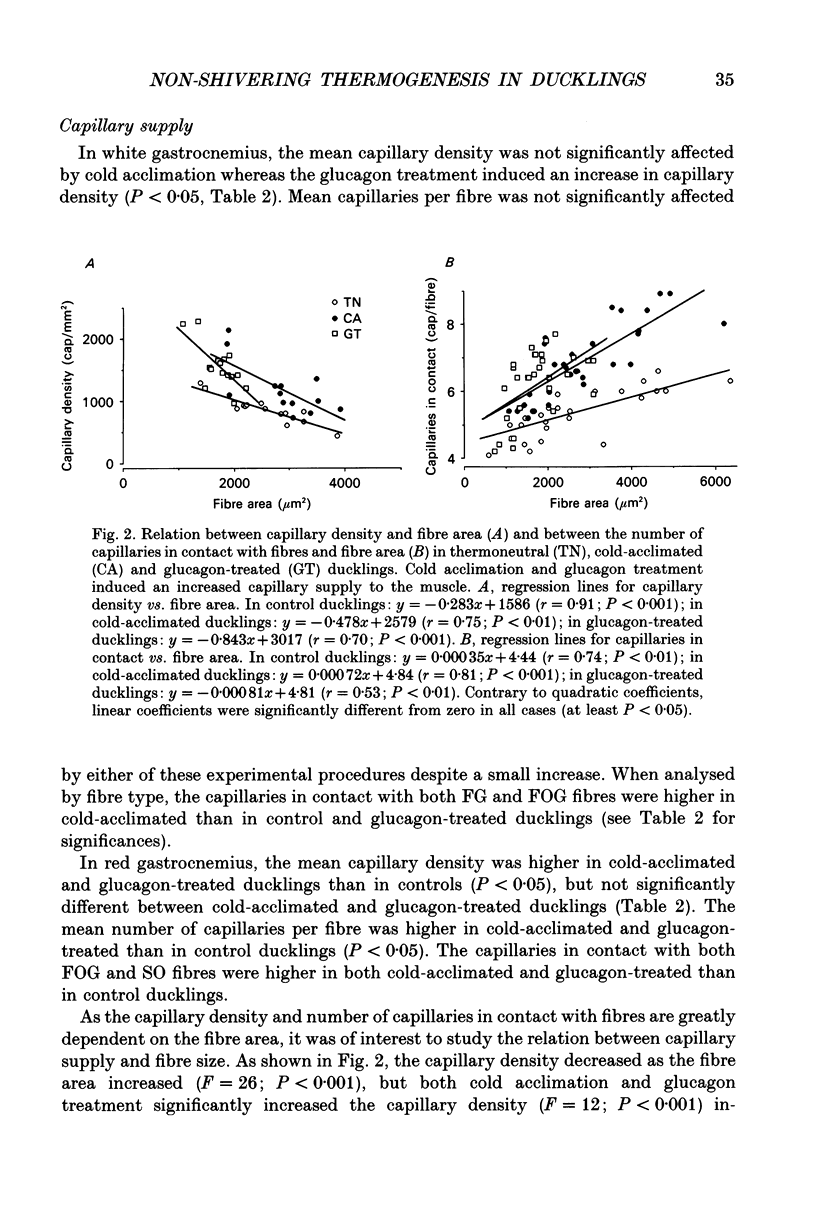
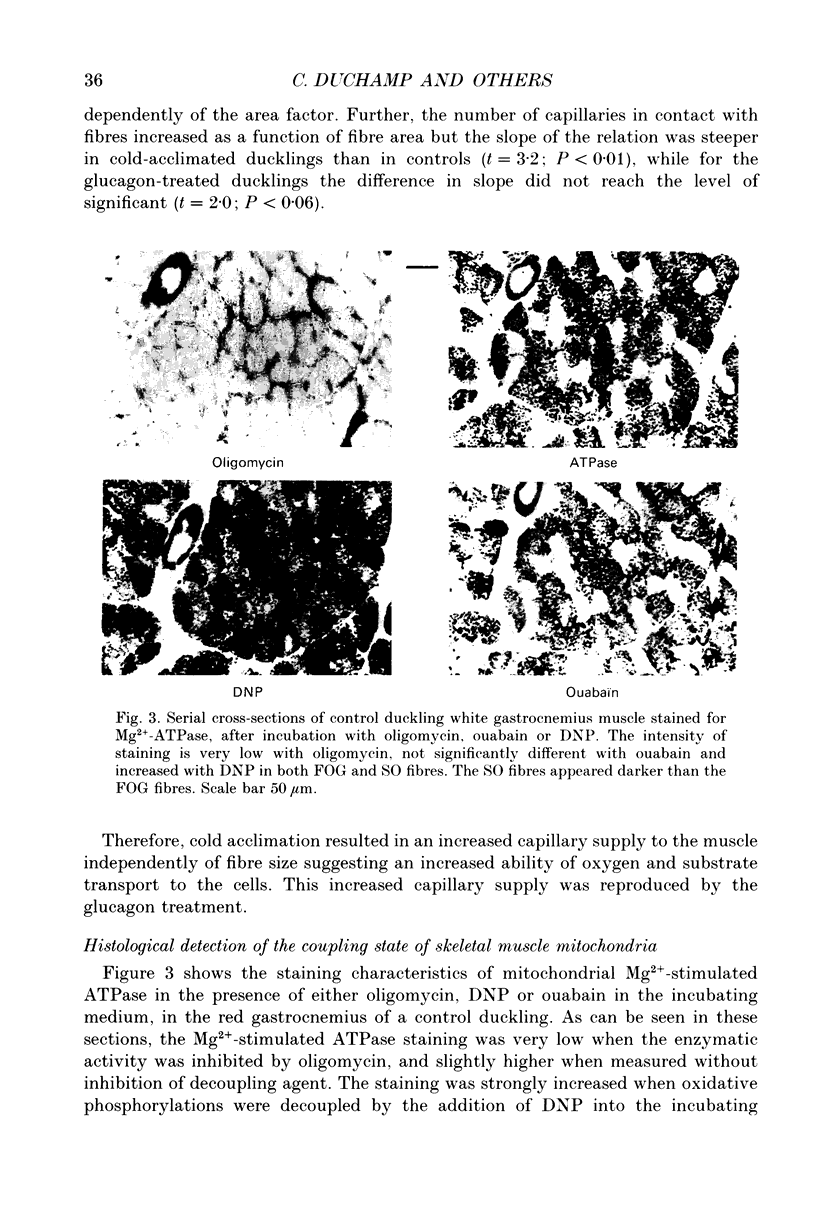
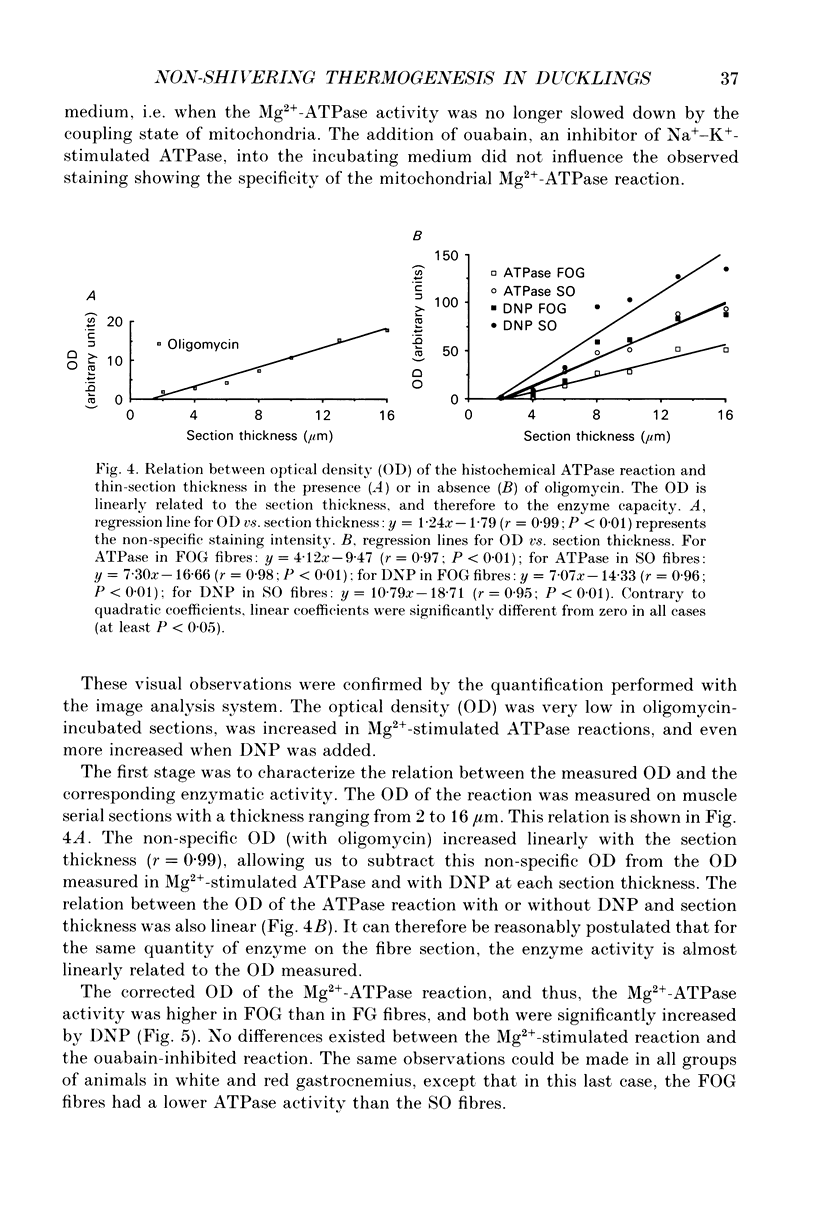
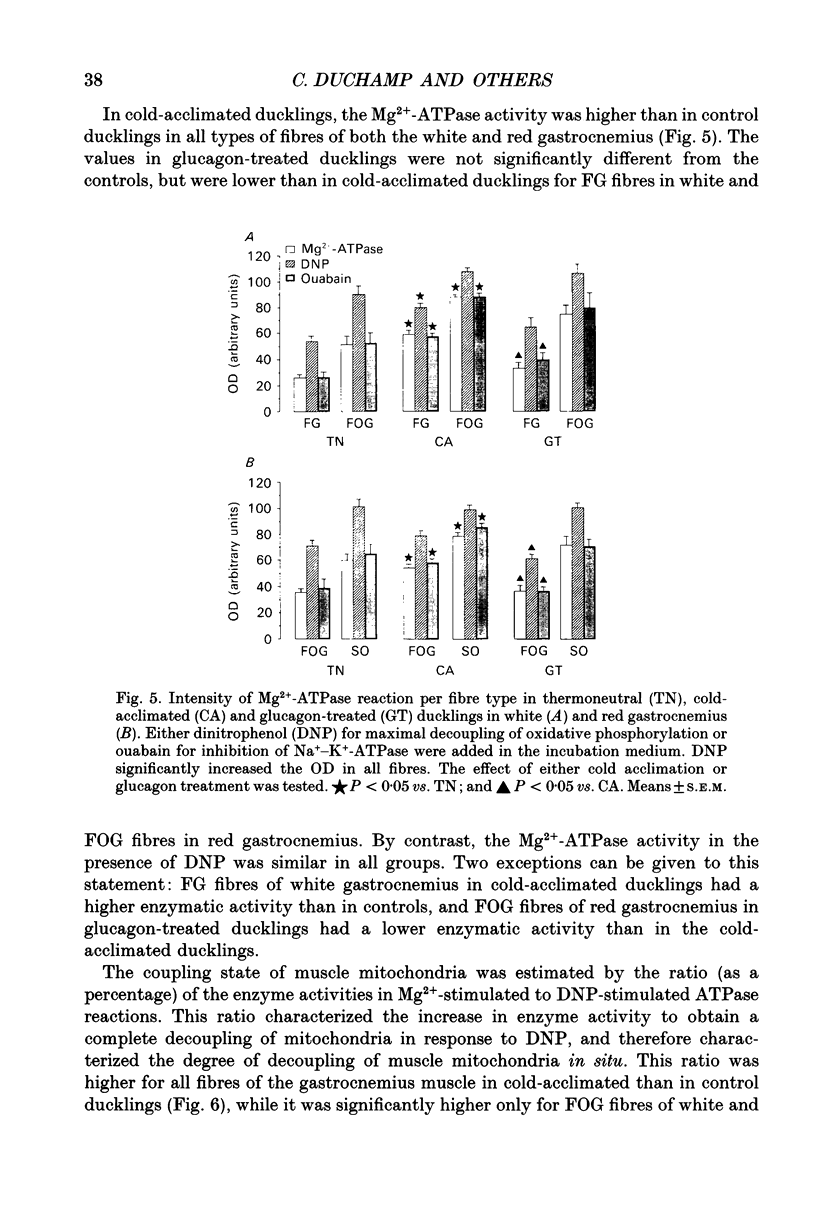
1. The histochemical characteristics of gastrocnemius muscle were investigated in 6-week-old cold-acclimated (5 weeks, 4 degrees C) and glucagon-treated (5 weeks, 25 degrees C, 103 nmol/kg I.P. twice daily) muscovy ducklings, two groups able to develop non-shivering thermogenesis in vivo. A comparison was made with thermoneutral controls (25 degrees C) of the same age. All animals were fed ad libitum. Fibre type, fibre area and capillary supply have been studied. Further, a quantitative histochemical method for mitochondrial Mg(2+)-ATPase activity was developed to characterize the mitochondrial coupling state in situ. 2. White gastrocnemius was composed of fast glycolytic (FG) and fast oxidative glycolytic (FOG) fibres, while red gastrocnemius contained FOG and slow oxidative (SO) fibres. In white gastrocnemius, the proportion of FG fibres was higher in glucagon-treated than in control or cold-acclimated ducklings. In red gastrocnemius, the proportion of SO fibres was higher in both cold-acclimated and glucagon-treated ducklings than in controls. The area of all fibres was generally lower in glucagon-treated than in other ducklings. 3. The capillary density was higher in both red and white components of the gastrocnemius muscle in cold-acclimated and glucagon-treated than in control ducklings, as a result of an increased number of capillaries around each fibre. 4. In all fibres, except the FG type in cold-acclimated ducklings, the staining intensity of the Mg(2+)-ATPase reaction was higher in cold-acclimated and glucagon-treated than in control ducklings whereas the staining intensity with maximal decoupling of oxidative phosphorylation by dinitrophenol was unchanged. This indicated a more loose-coupled state of mitochondria in situ in all fibres of cold-acclimated ducklings, and in FOG fibres of white gastrocnemius and SO fibres of red gastrocnemius in glucagon-treated ducklings. 5. These results indicated a higher oxidative metabolism of skeletal muscle in both cold-acclimated and glucagon-treated than in control ducklings, and for most of the parameters studied, a similarity between cold acclimation and glucagon treatment. Because of the higher loose-coupled state of muscle mitochondria in cold-acclimated and glucagon-treated than in control ducklings, the higher oxidative capacity of skeletal muscle in these ducklings could be used for heat production rather than ATP synthesis and account for muscular non-shivering thermogenesis.
Full text
PDF






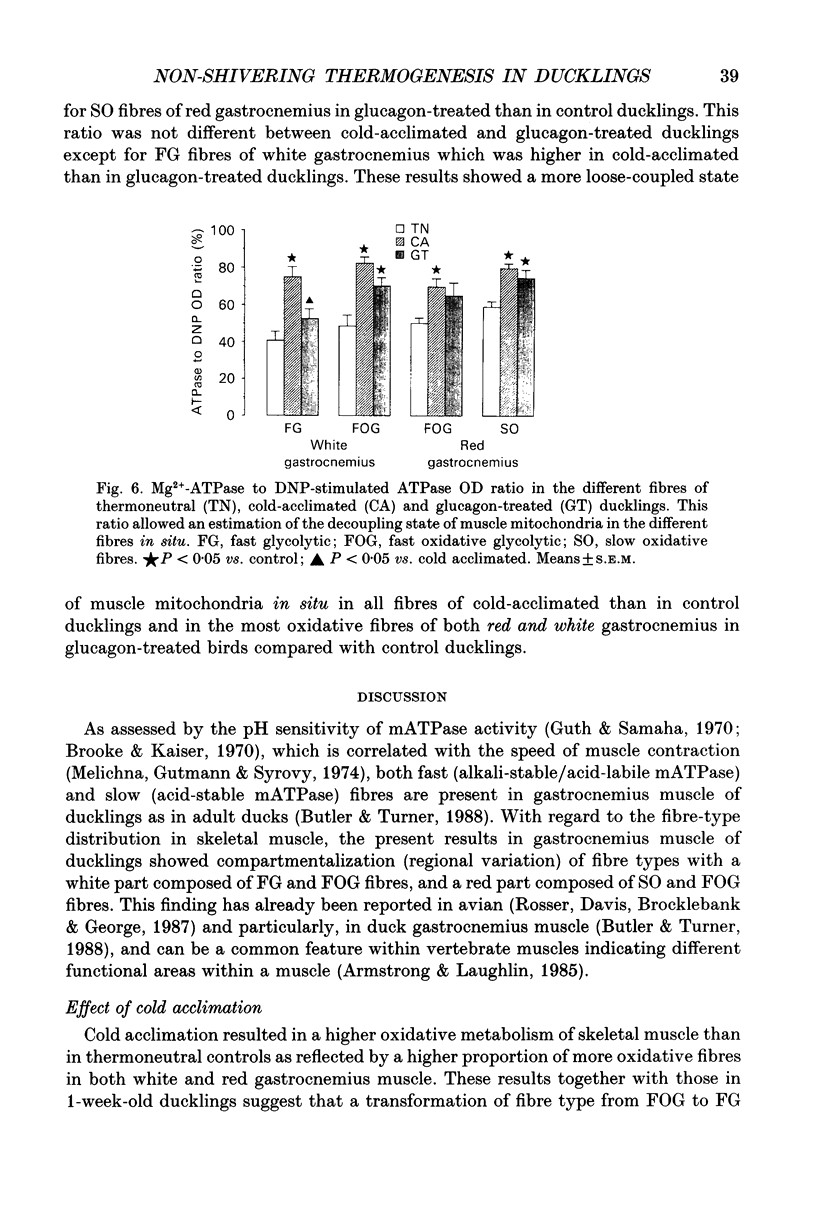






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977 Sep;270(3):677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. B., Laughlin M. H. Metabolic indicators of fibre recruitment in mammalian muscles during locomotion. J Exp Biol. 1985 Mar;115:201–213. doi: 10.1242/jeb.115.1.201. [DOI] [PubMed] [Google Scholar]
- Ashmore C. R., Doerr L. Postnatal development of fiber types in normal and dystrophic skeletal muscle of the chick. Exp Neurol. 1971 Mar;30(3):431–446. doi: 10.1016/0014-4886(71)90144-0. [DOI] [PubMed] [Google Scholar]
- Barré H., Bailly L., Rouanet J. L. Increased oxidative capacity in skeletal muscles from cold-acclimated ducklings: a comparison with rats. Comp Biochem Physiol B. 1987;88(2):519–522. doi: 10.1016/0305-0491(87)90337-3. [DOI] [PubMed] [Google Scholar]
- Barré H., Cohen-Adad F., Duchamp C., Rouanet J. L. Multilocular adipocytes from muscovy ducklings differentiated in response to cold acclimation. J Physiol. 1986 Jun;375:27–38. doi: 10.1113/jphysiol.1986.sp016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré H., Cohen-Adad F., Rouanet J. L. Two daily glucagon injections induce nonshivering thermogenesis in Muscovy ducklings. Am J Physiol. 1987 May;252(5 Pt 1):E616–E620. doi: 10.1152/ajpendo.1987.252.5.E616. [DOI] [PubMed] [Google Scholar]
- Barré H., Nedergaard J., Cannon B. Increased respiration in skeletal muscle mitochondria from cold-acclimated ducklings: uncoupling effects of free fatty acids. Comp Biochem Physiol B. 1986;85(2):343–348. doi: 10.1016/0305-0491(86)90010-6. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Turner D. L. Effect of training on maximal oxygen uptake and aerobic capacity of locomotory muscles in tufted ducks, Aythya fuligula. J Physiol. 1988 Jul;401:347–359. doi: 10.1113/jphysiol.1988.sp017166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauncey M. J., Ingram D. L. Influence of environmental temperature and energy intake on skeletal muscle respiratory enzymes and morphology. Eur J Appl Physiol Occup Physiol. 1988;58(3):239–244. doi: 10.1007/BF00417256. [DOI] [PubMed] [Google Scholar]
- Desplanches D., Mayet M. H., Sempore B., Flandrois R. Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol (1985) 1987 Aug;63(2):558–563. doi: 10.1152/jappl.1987.63.2.558. [DOI] [PubMed] [Google Scholar]
- Duchamp C., Barre H., Delage D., Rouanet J. L., Cohen-Adad F., Minaire Y. Nonshivering thermogenesis and adaptation to fasting in king penguin chicks. Am J Physiol. 1989 Oct;257(4 Pt 2):R744–R751. doi: 10.1152/ajpregu.1989.257.4.R744. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Henriksson J. The possible role of skeletal muscle in the adaptation to periods of energy deficiency. Eur J Clin Nutr. 1990;44 (Suppl 1):55–64. [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- Hoppeler H. Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med. 1986 Aug;7(4):187–204. doi: 10.1055/s-2008-1025758. [DOI] [PubMed] [Google Scholar]
- Howes G. A., Forbes J. M. A role for the liver in the effects of glucagon on food intake in domestic fowl. Physiol Behav. 1987;39(5):587–592. doi: 10.1016/0031-9384(87)90157-0. [DOI] [PubMed] [Google Scholar]
- Hudlická O. Development and adaptability of microvasculature in skeletal muscle. J Exp Biol. 1985 Mar;115:215–228. doi: 10.1242/jeb.115.1.215. [DOI] [PubMed] [Google Scholar]
- Jackson C. G., Sillau A. H., Banchero N. Fiber composition and capillarity in growing guinea pigs acclimated to cold and cold plus hypoxia. Proc Soc Exp Biol Med. 1987 May;185(1):101–106. doi: 10.3181/00379727-185-42524. [DOI] [PubMed] [Google Scholar]
- Katyare S. S., Fatterpaker P., Sreenivasan A. Effect of 2, 4-dinitrophenol (DNP) on oxidative phosphorylation in rat liver mitochondria. Arch Biochem Biophys. 1971 May;144(1):209–215. doi: 10.1016/0003-9861(71)90470-x. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O. Capillary tortuosity and degree of contraction or extension of skeletal muscles. Microvasc Res. 1987 Jan;33(1):98–117. doi: 10.1016/0026-2862(87)90010-0. [DOI] [PubMed] [Google Scholar]
- Meijer A. E., Vloedman A. H. The histochemical characterization of the coupling state of skeletal muscle mitochondria. Histochemistry. 1980;69(3):217–232. doi: 10.1007/BF00489769. [DOI] [PubMed] [Google Scholar]
- Melichna J., Gutmann E., Syrový I. Developmental changes in contraction properties, adenosine-triphosphatase activity and muscle fibre pattern of fast and slow chicken muscle. Physiol Bohemoslov. 1974;23(6):511–520. [PubMed] [Google Scholar]
- Mitchell P. Proton translocation mechanisms and energy transduction by adenosine triphosphatases: an answer to criticisms. FEBS Lett. 1975 Feb 1;50(2):95–97. doi: 10.1016/0014-5793(75)80465-0. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., SHIN W. Y., DRUCKER J. Mitochondrial localization of oxidative enzymes: staining results with two tetrazolium salts. J Biophys Biochem Cytol. 1961 Jan;9:47–61. doi: 10.1083/jcb.9.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G., Locke R. M. Thermogenic mechanisms in brown fat. Physiol Rev. 1984 Jan;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Pette D. J.B. Wolffe memorial lecture. Activity-induced fast to slow transitions in mammalian muscle. Med Sci Sports Exerc. 1984 Dec;16(6):517–528. [PubMed] [Google Scholar]
- Ricquier D., Barlet J. P., Garel J. M., Combes-George M., Dubois M. P. An immunological study of the uncoupling protein of brown adipose tissue mitochondria. Biochem J. 1983 Mar 15;210(3):859–866. doi: 10.1042/bj2100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. D., Kuzon W. M., Jr, Plyley M. J., Pynn B. R., McKee N. H. A histochemical method for the simultaneous demonstration of capillaries and fiber type in skeletal muscle. Stain Technol. 1987 Mar;62(2):85–92. doi: 10.3109/10520298709107973. [DOI] [PubMed] [Google Scholar]
- Rosser B. W., Davis M. B., Brocklebank J. R., George J. C. On the histochemical characterization and distribution of fast and slow muscle fibers in certain avian skeletal muscles. Acta Histochem. 1987;81(1):85–93. doi: 10.1016/s0065-1281(87)80081-8. [DOI] [PubMed] [Google Scholar]
- Sieck G. C., Lewis M. I., Blanco C. E. Effects of undernutrition on diaphragm fiber size, SDH activity, and fatigue resistance. J Appl Physiol (1985) 1989 May;66(5):2196–2205. doi: 10.1152/jappl.1989.66.5.2196. [DOI] [PubMed] [Google Scholar]
- Weigle D. S., Goodner C. J. Evidence that the physiological pulse frequency of glucagon secretion optimizes glucose production by perifused rat hepatocytes. Endocrinology. 1986 Apr;118(4):1606–1613. doi: 10.1210/endo-118-4-1606. [DOI] [PubMed] [Google Scholar]