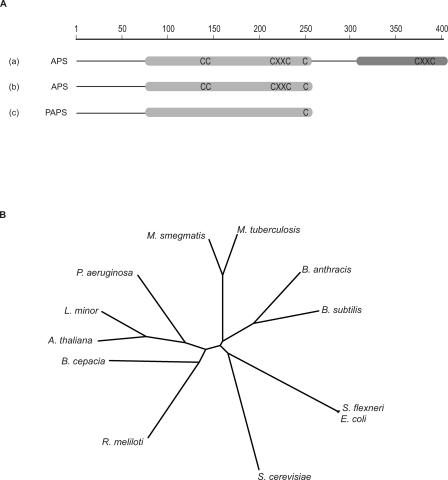
Figure 3. Domain Organization and Cysteine Conservation within the Sulfonucleotide Reductase Family.
(A) APS reductases from higher plants (group a) possess a reductase domain and a unique C-terminal domain with high homology to thioredoxin. Bacterial APS reductases (group b) lack this specialized domain, but share the cysteine motif -CC-X∼80-CXXC- present in the reductase domain. PAPS reductases (group c) lack this cysteine motif as well as the thioredoxin domain. All sulfonucleotide reductases have a cysteine at the end of the C terminus in the reductase domain. This residue is essential for catalysis.
(B) A dendrogram illustrating the sequence homology between enzymes within the sulfonucleotide reductase family. Each of the three subclasses of sulfonucleotide reductases is clearly delineated: APS reductases from higher plants with their unique C-terminal thioredoxin domain (A. thaliana and L. minor), bacterial APS reductases (M. tuberculosis, P. aeruginosa, and R. meliloti) and PAPS-reducing organisms (E. coli and S. cerevisae). Burkholderia cepacia, Shigella flexneri, B. subtilis, and Bacillus anthracis were included for comparison. The sequence alignment was performed using ClustalW and the tree was constructed with the Drawtree program [53].