Abstract
Implantable cardiac devices, including cardiac pacemakers, are not without risk for infection, carrying a mortality and morbidity of around 5–15%. Gram positive organisms are most common in 91% of cases, whereas gram negative organisms are less common, found in 2% of cases secondary to gram negative organisms other than Pseudomonas aeruginosa. Here, we present a rare case of the gram-negative organism Proteus mirabilis leading to a pacemaker site infection.
Keywords: Proteus mirabilis, Implantable cardiac device, Cardiac pacemaker, Ultrasound
1. Introduction
Implantable cardiac devices, including cardiac pacemakers (CP) and implantable cardioverter defibrillators (ICD), play an important role in the management and definitive treatment of various cardiac pathologies, including symptomatic bradycardia, sinus sick syndrome and high-grade second-and third-degree heart block, among others.1 Because pacemakers are considered foreign bodies, surgical placement of these devices is not without risk of infection.2 Mortality and morbidity secondary to infection of implanted devices is thought to range from 5 to 15%. The most observed infection is pacemaker pocket infection, although lead-associated endocarditis is also commonly observed.2,3 Most commonly, a pocket infection involves adjacent subcutaneous tissue encapsulating the device generator or the subcutaneous tissue surrounding the pacemaker leads.4 This differs from a systemic implantable cardiac device, which typically involves a transvenous segment and can lead to more serious complications, including endocarditis.5 If endocarditis is present, about half of cases may reveal a vegetation on echocardiography.5 Due to the complexity of device placement and severity of infection, treatment of device-related infections varies by disease severity and clinical presentation.6
Gram positive organisms are the most common pathogens leading to infection and found to be responsible in roughly 91% of cases.7 Coagulase negative staphylococcus was found to be the most common gram-positive organisms leading to pacemaker site infection, accounting for about 42% of infections.7 Mechanistically, these bacteria produce thick, multi-layered biofilms, making them more adherent to implantable devices.7 Other common gram-positive organisms responsible for infection include Staphylococcus aureus, Streptococcus, and Enterococcus.8 Gram negative organisms are less likely to be the causative organism of a pacemaker infection. Fakhro et al. described the prevalence of gram-negative organismal pacemaker infection as low as 9%.7 Garrigos et al. performed a retrospective review of all pacemaker-associated infections at Mayo Clinic from 1992 through 2015 and found only 5% of cases due to gram negative bacteria, with Pseudomonas aeruginosa being the most common causative organism.8 Furthermore, Tarakji et al. retrospectively reviewed 412 cases of infections involving implantable cardiac devices, finding that 2% of cases were due to gram negative organisms other than pseudomonas.9
These findings suggest that when presented with a cardiac device-related infection, although uncommon, it is important to maintain a high suspicion for gram negative causative organisms. Here, we present a case of a Proteus mirabilis pacemaker infection in a patient three months following permanent pacemaker placement.
2. Case presentation
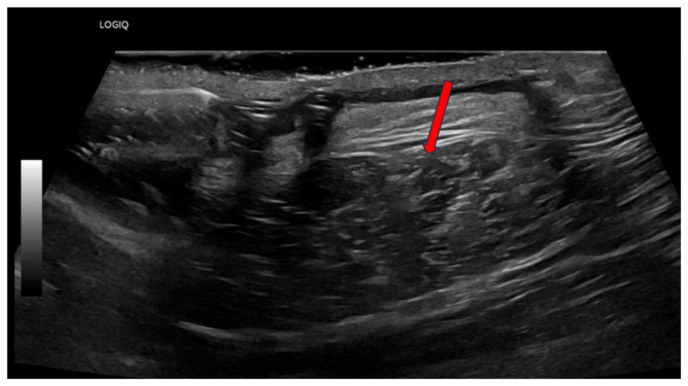
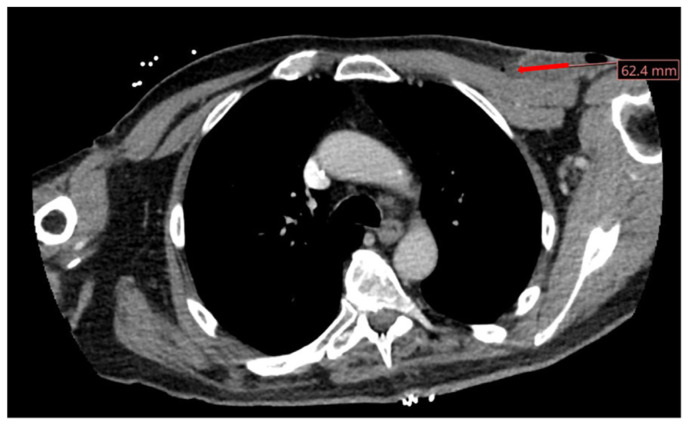
A 72-year-old male with a past medical history of complete heart block status post permanent pacemaker placement, diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease and bipolar disorder initially presented to the emergency department (ED) of our academic medical center as a transfer from an outside facility for concerns of a pacemaker infection. The patient initially had a permanent pacemaker placed 3 months prior. Initially, the patient endorsed erythema and chest discomfort reproducible with palpation. White blood cell count upon admission was within normal limits. However, C-reactive protein was elevated to 93.4 mg/L. MRSA nasal swab was negative. Soft tissue ultrasound was performed (Fig. 1). The patient was empirically started on vancomycin. The Infectious Disease service was consulted for antibiotic guidance and ultimately recommended continuation of vancomycin. Cardiac electrophysiology was consulted and recommended the removal of the transvenous dual pacemaker system with implantation of a Medtronic Micra AV leadless pacemaker. The patient underwent the procedure without complications. Aerobic and anaerobic cultures were obtained from the pacemaker pocket, pacemaker generator, left ventricular lead and atrial lead. A transthoracic echocardiogram (TTE) was obtained for an initial concern for endocarditis, which revealed a mildly depressed left ventricular systolic function of 45–50% with no obvious vegetation or significant regurgitation. The leadless pacemaker was further interrogated post-procedure with appropriate function. Aerobic and anaerobic cultures of the right atrial lead were positive for P. mirabilis. Blood cultures remained negative throughout the hospitalization. At the recommendation of infectious disease, the patient was transitioned from vancomycin to ceftriaxone. The Outpatient Parenteral Antimicrobial Therapy (OPAT) program was consulted prior to discharge with recommendations to continue ceftriaxone 2 g Q 24 h for a total antibiotic duration of 2 weeks. The patient was ultimately discharged from the hospital. Unfortunately, the patient developed purulent discharge from his left chest wall after discharge and returned to the ED 2 days later. In the ED, there was an absence of leukocytosis. Repeat blood cultures were collected and a CT of the chest with IV contrast was performed (Fig. 2). Infectious Disease recommended continuation of IV ceftriaxone. Cardiac electrophysiology was also consulted and recommended conservative management with a dry dressing. Cardiology believed his clinic presentation was likely secondary to rupture of a hematoma. Infectious Disease recommended the addition of doxycycline 100 mg b.i.d., which was added for additional Staphylococcus coverage given the degree of drainage present. The final plan was to continue the patient’s antimicrobial regimen of ceftriaxone and doxycycline at home for 2 weeks. The patient was discharged home and has been doing well since.
Fig. 1.
Soft tissue ultrasound of erythematous skin overlying pacemaker insertion site. Red arrow points to heterogenicity and cobblestoning of superficial soft tissue directly corresponding to area of overlying erythema.
Fig. 2.
CT scan demonstrating well-circumscribed left anterolateral chest wall fluid collection concerning for an abscess. There is associated evidence of a well-circumscribed collection of fluid and air with adjacent fat stranding.
3. Discussion
Here, we present a rare case of a pacemaker pocket infection secondary to the gram-negative organism P. mirabilis. Our patient initially presented with fluctuance on exam and erythema; however, he was without fever and leukocytosis. This clinical presentation is common among pacemaker pocket infections, as erythema and fluctuance may be present, but fever and systemic signs are typically absent.10 Systemic signs such as tachycardia, fever, leukocytosis and/or vital instability should be concerning for a systemic infection and require close clinical observation.11 Doring et al. analyzed predisposing factors placing individuals at risk for the development of a pacemaker-associated infection.3 Age >60, diabetes and a dual-chamber cardiac implantation device, all co-morbid conditions of our patient, were all associated with a relative risk of developing an associated infection of 2.5%, 2.1% and 1.5%, respectively. 3 End stage renal disease and four or more prior procedures for a cardiac device placement were both associate with the highest risk of infection with a relative risk of 8.7%.3 Our patient did not have these suggested common risk factors. Although often overlooked, underling co-morbid conditions in patients undergoing cardiac device placement must be considered when risk stratifying those patients at high-risk for post-procedure complications.3
Diagnosis of an implantable cardiac device infection requires both a clinical suspicion and a history of device placement. In such cases, blood cultures should be obtained prior to administering antimicrobials. 3 In cases such as ours without overt bacteremia, blood cultures may be negative; however, this should not obliviate the need for antimicrobial therapy. Transesophageal echocardiography (TEE) should also be performed at time of suspected infection, especially in the presence of bacteremia, as the presence or absence of valve or lead vegetation will affect antimicrobial duration.6,12–14 In cases where a valvular vegetation is found, the cardiac device should be removed, followed by 4–6 weeks of antimicrobial therapy; however, in the event a cardiac device lead vegetation is found, 2–4 weeks of antimicrobial therapy is preferred.6,12–14 In both cases, the device is recommended to be extracted. 6,12–14 Our patient remained hemodynamically stable and blood cultures were negative throughout hospital admission. Because of this, a less invasive TTE was favored with good visualization of valvular anatomy. TEE was also deferred in the setting of gram-negative bacteremia. If blood cultures remained positive and a gram-positive bacteremia was suspected with a high probability for a valvular vegetation, a TEE would have been preferred. In such cases, extraction and empiric antimicrobial therapy is recommended for 2 weeks.6,12–14
Empiric antimicrobial therapy in a hemodynamically stable patient should target gram positive organisms, such as Methicillin resistant staphylococcus aureus (MRSA) and Staphylococcus epidermidis, as these are the most common organisms leading to implantable cardiac device-related infections.12 Common empiric treatment includes vancomycin.12 In presentations of hemodynamically instability, empiric therapy should also include cefepime or piperacillin-tazobactam.12 Interestingly, as noted above, P. mirabilis was found to have grown on the patient’s atrial pacemaker lead. Growth of uncommon organisms should prompt clinicians to explore other sites of infectious origin. Upon further chart review, our patient was found to have a prior Proteus gallbladder abscess. Proteus is a common intraabdominal organism that can be colonized after abdominal surgery and/or urinary tract infection.15 It is hypothesized that Proteus may have colonized the patient after manipulation of his gallbladder fossa for his abscess. Nandhakumar et al. described a pacemaker pocket infection that was found to be secondary to Pseudomonas aeruginosa in a patient found to have gram negative septicemia. This case, along with ours, raises the consideration of obtaining a TTE even in the setting of gram negative septicemia.16 In addition, Berkefeld et al. described a pacemaker pocket infection secondary to Clostridium difficile, thought to be due to disruption in mucosal barrier function leading to translocation.17 Similarly, foreign bodies, such as aortic grafts, have been reported to be infected secondary to C. difficile in those with diverticulitis.17 Infection was thought to be due to hematogenous spread, such as our case.
Advancements in cardiac devices have reduced the risk of pacemaker-related infections, thought to be secondary to the absence of transvenous leads and the need for a subcutaneous pocket.18 Interestingly, clinical trials found an absence of infection in those with a leadless pacemaker, even in those deemed “high risk” for infection, as noted above.18 Leadless pacemakers may be a viable option for those high risk for cardiac device infection. As cardiac device-related infections secondary to gram negative bacteria are less common, our case highlights the importance of maintaining a broad differential when presented with clinical suspicions of an implantable cardiac device infection and recognizing a rare cause of a common device-related infection.
4. Conclusion
Implantable cardiac devices, including pacemakers, are essential for treatment of certain pathologies, including sinus bradycardia and complete heart block, among others. Gram positive bacteria are the most causal organisms; however, in rare cases, gram negative organisms can also be the causative pathogen. Gram negative organisms other than Pseudomonas aeruginosa have been reported in approximately 2% of cases.9 Our case highlighting a P. mirabilis implantable cardiac device raises the importance of maintaining a broad differential when presented with such infections.
Abbreviation
- CP
Cardiac pacemakers
- ICD
Implantable cardioverter defibrillators
- TTE
Transthoracic echocardiogram
- ED
Emergency department
- TEE
Transesophageal echocardiography
- MRSA
Methicillin resistant staphylococcus aureus
Footnotes
Conflict of interest: Each author certifies that he/she, a member of his or her immediate family, has no commercial association (i.e., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted manuscript. The authors have not used Generative Artificial Intelligence or Artificial Intelligence associated technologies.
Funding: None.
References
- 1. Al-Khatib SM. Cardiac implantable electronic devices. N Engl J Med. 2024;390:442–454. doi: 10.1056/NEJMra2308353. [DOI] [PubMed] [Google Scholar]
- 2. Goutam D. A study on pacemaker pocket infection. J Cardiol Cardiovasc Med. 2020;5:56–59. [Google Scholar]
- 3. Döring M, Richter S, Hindricks G. The diagnosis and treatment of pacemaker-associated infection. Dtsch Arzteblatt Int. 2018;115:445–452. doi: 10.3238/arztebl.2018.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Da Costa A, Lelievre H, Kirkorian G, et al. Role of the preaxillary flora in pacemaker infections: a prospective study. Circulation. 1998;97:1791–1795. doi: 10.1161/01.cir.97.18.1791. [DOI] [PubMed] [Google Scholar]
- 5. Duval X, Selton-Suty C, Alla F, et al. Endocarditis in patients with a permanent pacemaker: a 1-year epidemiological survey on infective endocarditis due to valvular and/or pacemaker infection. Clin Infect Dis. 2004;39:68–74. doi: 10.1086/421493. [DOI] [PubMed] [Google Scholar]
- 6. Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551. doi: 10.1016/j.hrthm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 7. Fakhro A, Jalalabadi F, Brown RH, Izaddoost SA. Treatment of infected cardiac implantable electronic devices. Semin Plast Surg. 2016;30:60–65. doi: 10.1055/s-0036-1580733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Esquer Garrigos Z, George MP, Vijayvargiya P, et al. Clinical presentation, management, and outcomes of cardiovascular implantable electronic device infections due to gram-negative versus gram-positive bacteria. Mayo Clin Proc. 2019;94:1268–1277. doi: 10.1016/j.mayocp.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 9. Tarakji KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm. 2010;7:1043–1047. doi: 10.1016/j.hrthm.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 10. Chua JD, Wilkoff BL, Lee I, et al. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000;133:604–608. doi: 10.7326/0003-4819-133-8-200010170-00011. [DOI] [PubMed] [Google Scholar]
- 11. Esquer Garrigos Z, George MP, Khalil S, et al. Predictors of bloodstream infection in patients presenting with cardiovascular implantable electronic device pocket infection. Open Forum Infect Dis. 2019;6:ofz084. doi: 10.1093/ofid/ofz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandoe JAT, Barlow G, Chambers JB, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint working party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE) J Antimicrob Chemother. 2015;70:325–359. doi: 10.1093/jac/dku383. [DOI] [PubMed] [Google Scholar]
- 13. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESCguidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European association for Cardio- Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 14. Baddour LM, Garrigos ZE, Sohail MR, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 15. Hamilton AL, Kamm MA, Ng SC, Morrison M. Proteus spp. as putative gastrointestinal pathogens. Clin Microbiol Rev. 2018;31:e00085–17. doi: 10.1128/CMR.00085-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nandhakumar B, Menon T, Ravishankar G, Shanmugasundaram S. Pacemaker pocket infection associated with septicemia caused by Pseudomonas aeruginosa. Int J Infect Dis. 2008;12:107–108. doi: 10.1016/j.ijid.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 17. Berkefeld A, Berger FK, Gartner BC, et al. Clostridioides (Clostridium) difficile pacemaker infection. Open Forum Infect Dis. 2020;7:ofaa487. doi: 10.1093/ofid/ofaa487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El-Chami MF, Bonner M, Holbrook R, et al. Leadless pacemakers reduce risk of device-related infection: review of the potential mechanisms. Heart Rhythm. 2020;17:1393–1397. doi: 10.1016/j.hrthm.2020.03.019. [DOI] [PubMed] [Google Scholar]