Abstract
Objective
To identify the best evidence on the efficacy of treatment interventions for inclusion body myositis (IBM) and to describe their safety.
Methods
Systematic review of randomised controlled trials (RCTs) of pharmacological treatments of adults with IBM, conducted according to the Cochrane Handbook, updating a previous Cochrane review. The search strategy was run on Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL, MEDLINE and EMBASE, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform. Assessment of risk of bias, data extraction and synthesis were performed independently by two reviewers. Data pooled in statistical meta-analyses, if possible.
Results
From a total of 487 records, 48 were selected for full-text review, 14 fulfilled the inclusion criteria, but only 2 RCTs were included in meta-analyses due to clinical heterogeneity (different drug interventions or dosages). Treatments included various immunosuppressive and immunomodulatory agents, alongside interventions modulating muscle growth and protein homoeostasis. Efficacy was assessed across multiple outcomes, namely muscle strength, physical function, mobility and muscle trophicity. Trials of methotrexate (MTX), intravenous immunoglobulin, interferon beta-1a and MTX, MTX and anti-T-lymphocyte immunoglobulin, oxandrolone, MTX and azathioprine, bimagrumab, arimoclomol, and sirolimus provided low-quality to high-quality evidence of having no effect on the progression of IBM.
Conclusions
Drug interventions for IBM were not effective for most of the outcomes of interest. We observed inconsistency of outcome measures across trials. More RCTs are needed, of adequate size and duration, and using a standardised set of outcome measures.
Keywords: Antirheumatic Agents; Autoimmunity; Biological Therapy; Treatment; Outcome Assessment, Health Care
WHAT IS ALREADY KNOWN ON THIS TOPIC
Inclusion body myositis (IBM) is the most prevalent muscle-wasting disease in people over the age of 45, with no currently effective treatment.
Recent large clinical trials highlight the need to update the evidence on the effects of pharmacological interventions in IBM.
WHAT THIS STUDY ADDS
This systematic review (SR) highlights the need for a core outcome set to consistently assess treatment response and disease progression in IBM, potentially enabling studies to produce more precise results.
Pharmacological interventions for IBM remain ineffective for most of the outcomes of interest, though evidence regarding their safety is reassuring.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This SR updates the efficacy and safety of treatment interventions for IBM, incorporating findings from recent large clinical trials targeting novel pathways.
This SR encourages further studies exploring the efficacy of interventions in IBM, where evidence for management is still scarce.
Introduction
Inclusion body myositis (IBM) is the most common muscle-wasting disease in people older than 45 years and is associated with progressive proximal and distal limb muscle atrophy and weakness.1,4 Early clinical features on physical examination are the preferential involvement of finger flexion and knee extension muscle groups, with neck flexion and ankle dorsiflexion also being frequently affected. Dysphagia can be a presenting feature and occurs frequently during the disease course. The disease progresses slowly but relentlessly over time, interfering with all activities of daily living, leading to severe disability, loss of quality of life, work impairment and premature death.1,9
IBM is a rare disease and the epidemiology varies between and within countries, with an estimated overall prevalence of 46 per million (increasing to 97 per million in people older than 30 years and 139 per million in people older than 50 years).10 11 However, the prevalence is likely underestimated as the condition is often misdiagnosed and a mean diagnostic delay of around 5 years has been reported.23 5,9 The estimated annual US cost of care for IBM is US$3500012; this includes the total healthcare costs and resource utilisation for all services, such as inpatient visits, emergency room visits, outpatient office visits, durable medical equipment claims, skilled nursing facility visits, home health agency visits, hospice care and prescriptions.
The pathophysiology is complex, remains poorly understood and is considered to involve an interaction between inflammatory and degenerative pathways.3 13 14 The degenerative theory of IBM hypothesises that the disease is caused by ageing of muscle fibres associated with accumulation and aggregation of misfolded, ubiquitinated, multiple-protein aggregates in genetically susceptible people. Accumulation of these protein aggregates within muscle fibres is thought to trigger an inflammatory/immune response as a secondary outcome of muscle degeneration.3 6 Conversely, given the inflammatory features on muscle biopsy such as marked CD8+T cell infiltration and major histocompatibility complex class I and II upregulation, the identification of genetic autoimmunity associations,4 15 the association with antibodies against cytosolic 5́-nucleotidase 1A (cN1A),16,18 and the identification of a highly differentiated T-cell population known to be relatively resistant to apoptosis and other mechanisms of immunosuppression, a primarily autoimmune aetiology for IBM has been suggested, with the degenerative features hypothesised as being a secondary component.9 19
Treatment options have attempted to target inflammatory, degenerative, and atrophic features of IBM, with large clinical trials having been recently conducted5 6 8 since the last systematic review (SR) took place.1 Since there was no recent SR investigating the efficacy of treatment interventions for IBM, and considering the need to expand the body of knowledge in light of new and large clinical trials, we performed an SR that aimed to identify the best evidence on the efficacy of treatment interventions for IBM and to describe their safety, if reported, in the included studies.
Methods
This SR was conducted according to the Cochrane Handbook20 and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.21
As this is an update of a Cochrane SR, the research team followed the same protocol as the previous Cochrane SR.1 The review was not registered in PROSPERO.
The outlined research questions were:
Which pharmacological interventions are efficacious in improving outcomes in people with IBM?
Which pharmacological interventions are safe in improving outcomes in people with IBM?
We only focused on pharmacological interventions because new clinical trials have emerged in this area. We did not include trials of exercise or management of dysphagia, as these are subjects of other Cochrane reviews with no significant updates expected since the last review.22
These questions were framed and structured using the ‘Patients, Intervention, Comparator or Control, Outcome, Type of study' format, as follows:
Participants
A study was eligible for inclusion if the included participants were adults (aged 18 years or over) with a diagnosis of IBM fulfilling any internationally accepted IBM classification criteria.23,26 We specifically excluded people with familial IBM and hereditary inclusion body myopathy, but we included people who had connective tissue and autoimmune diseases associated with IBM, which may or may not have been described in trials. Studies describing information regarding people with IBM associated with other concomitant diseases were summarised separately and by subgroups, whenever possible.
Interventions
Regarding the eligible interventions, all pharmacological interventions were included. Pharmacological interventions were classified as medicinal products in accordance with the EU Directive 2001/83/EEC (EU 2001), which states: ‘any substance or combination of substances which may be used in or administered to human beings either with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis’.27 This may include interventions such as immunosuppressive or immunomodulatory agents including glucocorticoids, azathioprine, methotrexate (MTX), ciclosporin, cyclophosphamide, intravenous immunoglobulin, leukapheresis, plasma exchange and targeted therapies such as those using monoclonal antibodies, among others.
Comparator or control
The comparator was placebo or usual care (standard care). Studies without a comparator were excluded.
Context
There were no contextual constraints.
Outcomes
Regarding outcomes, the core concepts were change in muscle strength (eg, Manual Muscle Testing (MMT), Quantitative Muscle Testing (QMT) using Maximal Voluntary Isometric Contraction Testing (MVICT) or hand-held dynamometry) and muscle trophicity (eg, Thigh Muscle Volume (TMV), lean body mass (LBM)). Based on the impact of the condition, we additionally included changes in measures of mobility, endurance or physical function, such as the 6 min Walking Distance (6MWD), Timed Up and Go Test (TUG) or its modified version (mTUG) and Inclusion Body Myositis Functional Rating Scale (IBMFRS), among others. To analyse the safety of interventions, the absolute number of adverse events was considered.
Type of study
Only SRs and randomised controlled trials (RCTs) or controlled clinical trials were eligible because they are considered the most robust study designs and represent the strongest evidence.28 The studies integrating SRs were extracted for joint analysis with the remaining primary studies. This was done to provide additional reassurance that no relevant articles were overlooked. In this regard, the SRs themselves were not included and evaluated.
Search strategy and study selection
A search strategy was run in Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL, MEDLINE, EMBASE, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform, from 1 September 2014 (end date of the search strategy of the last SR)1 to 31 January 2023. Studies published in English, French, Portuguese, Spanish and Turkish language were considered for inclusion. Details on complete search strategies are provided in online supplemental material S1.
All identified citations were uploaded into an EndNote V.X9 (Clarivate Analytics, PA, USA) library and the duplicates were removed. Titles and abstracts were screened by two independent reviewers (EJFS and BF) to assess eligibility criteria. The full articles were retrieved for all studies that met or had insufficient information to assess the inclusion criteria, and two reviewers (EJFS and BF) independently examined them in detail. Any disagreements between the reviewers were resolved through discussion or adjudication by a third reviewer (PMM). The study selection was performed using Rayyan.
Risk of bias (quality) assessment
Two reviewers (EJFS and BF) assessed the risk of bias in each included study using the Cochrane Collaboration’s tool for RCTs.29 Any disagreements between the reviewers were resolved through discussion or adjudication by a third reviewer (PMM). The Cochrane’s risk of bias tool (RoB-1) was used since this SR is an update and because it was the previously used tool. This procedure is in accordance with the standardised methods of the Cochrane Handbook.20
Data extraction and synthesis
Data were extracted from the selected reports by the same two independent reviewers (EJFS and BF), and disagreements were discussed until consensus was achieved, or with adjudication by the third reviewer (PMM), whenever necessary. Authors of papers were contacted to request missing or additional data, where required.
Studies were pooled for statistical meta-analysis using Review Manager V.5.2.8. and SPSS Statistics, V.28 (IBM), if the needed statistics were available. Effect sizes were expressed as ORs (for dichotomous data) or mean differences (MD), and their 95% CIs were calculated. MD is the difference between effect estimates for intervention and control on a specific scale. Because pooling of the MD from individual RCTs is done after weighting the values for precision, this pooled MD is also known as the weighted MD. From a clinical perspective, interpretation requires knowing the minimally important difference for the outcome.20 We imputed SD where necessary according to sections 6.5.2.2 and 6.5.2.3 of the Cochrane Handbook.20 Heterogeneity was assessed statistically using the standard χ2 and I² tests. For a value of I² equal to 0%, we assume no heterogeneity between studies (homogeneity); around 25%, low heterogeneity; around 50%, moderate heterogeneity; and around or greater than 75%, high heterogeneity.30 Statistical analyses were performed using random effects models only in the presence of moderate to high heterogeneity (I²>50%) and, in their absence, fixed effect models were used instead.31 32 Where statistical pooling was not possible, the findings were presented in narrative form, including tables and figures, where appropriate. Sensitivity analyses were conducted to test decisions made, if appropriate.
Results
Out of a total of 487 records, 48 were selected for full-text review, and 14 studies fulfilled the inclusion criteria and were included in this SR. Of these, only two RCTs were included in the meta-analysis due to clinical heterogeneity of the remaining studies (different drug interventions or dosages). There was no need to contact the authors of the papers to request additional information. The results of the searches are shown in a flow diagram (figure 1).
Figure 1. Flow chart of the study selection and inclusion process.
Methodological quality
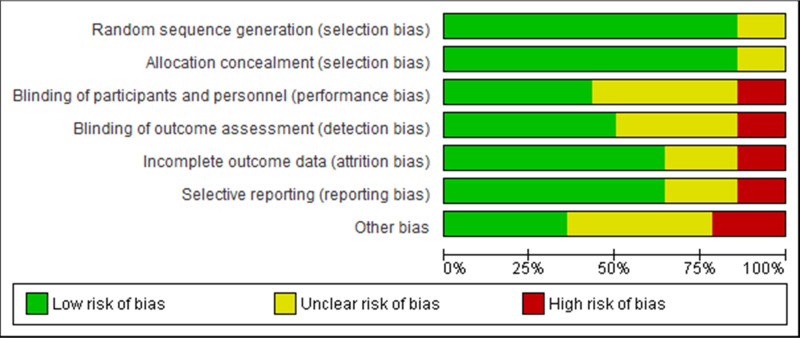
The critical appraisal results for each study are summarised in figure 2 and online supplemental material S2. There was agreement among the reviewers to include all the studies that were appraised. The more recent RCTs included in this SR update were of high quality.5 6 8 33 34 Five RCTs were of moderate quality.35,39 Finally, four RCTs were of low quality, indicating a high risk of bias.40,43 All but two of the RCTs complied with a random sequence generation and allocation concealment. There was an unclear risk of bias or high bias in ~57% of the RCTs, for blinding of participants and personnel and blinding of outcome assessment. For incomplete outcome data and selective reporting, an unclear risk of bias or high bias was observed in ~43% of the RCTs. For other biases, an unclear risk of bias or high bias was observed in ~64% of the RCTs. Overall, the included RCTs provide low to high-quality evidence, with notable improvement observed in the more recent studies.
Figure 2. Risk of bias summary graph for included clinical trials. Review authors’ judgements about each risk of bias item presented as percentages across all included studies using the Cochrane RoB tool. RoB, risk of bias.
Characteristics of included studies and interventions
Study characteristics are detailed in online supplemental material S3. Pharmacological interventions included various immunosuppressive or immunomodulatory agents alongside interventions aimed at modulating muscle growth or protein homoeostasis, namely MTX alone,33 intravenous immunoglobulin,35 40 41 interferon beta-1a and MTX,36 37 anti-T-lymphocyte immunoglobulin and MTX,42 azathioprine and MTX,43 oxandrolone,38 bimagrumab,8 34 arimoclomol6 39 and sirolimus.5 The summary of findings, integrating all included RCTs, along with the interventions and their impact on outcomes, is presented in table 1. The follow-up periods varied from 335 40 to 206 months, with 12 months being the most common follow-up period.5 8 33 39 41 42 Overall, we found that pharmacological interventions were not effective for most of the outcomes of interest and had no effect on the progression of IBM.
Table 1. Summary of findings.
| Intervention | Number of RCTs | Outcome | Impact | References |
| Methotrexate (MTX) vs placebo | 1 | QMT; MMT | No difference | 33 |
| Intravenous Immunoglobulin vs placebo | 2 | MMT; QMT (MVICT); NSS | No difference | 35 40 41 |
| 1 | NSS | Improvement | 41 | |
| Interferon beta-1a+MTX vs placebo | 2 | NSS; Average grip strength; QMT (MVICT); LBM; MMT (MRC) | No difference | 36 37 |
| Anti-T-lymphocyte Immunoglobulin+MTX vs MTX | 1 | QMT | Improvement | 42 |
| Oxandrolone vs placebo | 1 | QMT (MVICT); MMT (MRC) | No difference | 38 |
| Azathioprine+MTX vs MTX | 1 | MMT | Unclear | 43 |
| Bimagrumab vs placebo | 2 | LBM; TMV | Improvement | 8 34 |
| QMT; 6MWD; mTUG | No difference | |||
| Arimoclomol vs placebo | 2 | IBMFRS; MMT; mTUG; 6MWD;Hand grip strength | No difference | 6 39 |
| Sirolimus vs placebo | 1 | 6MWD; HAQ-DI | Improvement | 5 |
| IBMFRS; QMT (Grip strength, Knee extension strength) | No difference |
HAQ-DIHealth Assessment Questionnaire - Disability IndexIBMFRSInclusion Body Myositis Functional Rating ScaleLBMlean body massMMTmanual muscle testingMRCMedical Research Council ScalemTUGmodified Timed Up and Go TestMVICTMaximal Voluntary Isometric Contraction Testing6MWD6 min Walking DistanceNSSNeuromuscular Symptom ScoreQMTQuantitative Muscle TestingRCTsrandomised controlled trialsTMVthigh muscle volume
Regarding the safety of pharmacological interventions, most studies reported that they were well tolerated or similar to placebo.56 8 33,43
Meta-analysis and narrative synthesis
Meta-analysis of results to assess the efficacy of the different pharmacological interventions was only possible in two RCTs (for interferon beta-1a vs placebo comparison) due to the marked differences between the included RCTs (different interventions, different modes of administration, different dosages and different follow-ups, among others; resulting in a highly irreconcilable clinical heterogeneity). The results of the meta-analyses were carried out for four outcomes (average grip strength, composite MVICT score, dual energy X-ray absorptiometry (DEXA) LBM and MMT) but none revealed statistically significant differences (figure 3; p>0.05). In addition, we carried out meta-analyses to determine the OR of serious adverse events of the different pharmacological interventions. The summary of the meta-analyses was grouped into a single forest plot (figure 4). The results of the meta-analyses and the narrative summary (online supplemental material S3) show that the pharmacological interventions were well tolerated or similar to placebo in terms of adverse events and serious adverse events. Only one exception was observed for sirolimus (OR 2.16, 95% Cl 0.64 to 7.26).
Figure 3. Meta-analyses for Interferon beta-1a versus placebo. The values shown are mean differences in the different identified outcomes and their 95% CIs. DEXA, dual energy X-ray absorptiometry; MMT, manual muscle testing; MVICT, Maximal Voluntary Isometric Contraction Testing.
Figure 4. Meta-analyses summary of the OR of serious adverse events (SAEs). The values shown are OR and their 95% CIs from the comparison of the identified pharmacological intervention versus placebo. An OR greater than 1 indicates that the serious adverse event is more likely to occur. IVIg, intravenous immunoglobulin.
Discussion
This SR provides evidence that pharmacological interventions are largely ineffective for most outcomes of interest and disease progression in people with IBM. However, a few studies indicated some beneficial effects for certain interventions, including intravenous immunoglobulin,41 anti-T-lymphocyte immunoglobulin plus MTX,42 bimagrumab8 34 and sirolimus,5 on some assessed outcomes. These findings, however, have not been consistently replicated, nor was there uniformity across the outcome measures. Regarding safety, most trials reported that the interventions were well tolerated, with adverse events generally mild and similar to those observed with placebo.
Due to the lack of an agreed-on core outcome set in IBM,44 45 a wide range of different assessments—including muscle strength, endurance, physical function and quality of life assessments—have been evaluated as primary or secondary measures in studies, making integration into meta-analyses challenging. Meta-analyses were only possible in two RCTs and found no significant differences in primary or secondary outcomes, including mean grip strength, MVICT composite score, DEXA LBM and MMT. In most studies, muscle strength was the primary outcome, but in two recent RCTs of high quality with the highest number of patients, endurance (6MWD)8 and physical function (IBMFRS)6 were the primary outcomes. Overall, muscle strength testing, such as QMT5 33 35 38 42 and MMT,35 40 41 43 and safety and tolerability,36 37 39 were the most common primary outcome measures. These were followed by laboratory tests,42 43 NSS,41 open muscle biopsy findings,42 assessment of activities of daily living,43 TMV measured by MRI,34 and the already mentioned 6MWD8 and IBMFRS score,6 each used in one study. These discrepancies in primary outcome measures highlight the need for a core set to assess treatment efficacy in IBM.44,53
Given IBM’s slow progression,3 determining the optimal time to assess treatment efficacy and the overall follow-up duration is critical, though no consensus exists. The follow-up periods in studies included in this review varied from 335 40 to 206 months, with 12 months being the most common follow-up period.5 8 33 39 41 42
The pathogenesis of IBM remains poorly understood,3 13 14 but recent advances have led to trials of new treatment strategies targeting various pathways.5 6 8 Interventions in this review have targeted immunosuppression,533 35,37 40 muscle protein degradation,6 39 and muscle wasting and atrophy.8 34 38 These results provide a foundation for future studies with more clearly defined endpoints and treatment targets. Additionally, investigating alternative pathways (eg, mitochondrial changes) or using combination therapies targeting multiple pathways6 could be explored in the future.
Following the Sirolimus (Rapamycin) phase 2 clinical trial in IBM with some promising secondary outcome results,5 a phase 3 clinical trial with the same drug is ongoing (NCT04789070). Sirolimus inhibits the mammalian target of rapamycin pathway which has various effects on cell metabolism, including promoting autophagy.5 Furthermore, it has a role in immunomodulation by upregulating Tregs and the suppression of effector CD8/CD4+T cells. Therefore, the drug may have a multifaceted effect on IBM.
The clonal expansion and replacement of naïve or early effector memory T cells (TEM1 and TEM2 cells) in both the blood and muscle tissue of IBM patients not only provide evidence of chronic antigen-driven T cell activation9 but also represent immunosenescence.54 While the specific antigen responsible for triggering autoreactivity of CD8+and CD4+ T cells remains elusive, the enduring presence of the highly differentiated effector memory and cytotoxic CD4+ (CD4+CD28-) and CD8+T cells (CD8+CD28-, CD8+CD244+, CD8+CD57+, CD8+Killer cell lectin-like receptor G1 (KLRG1)+) infiltrating the muscles suggests evasion from the regulatory immune mechanisms that typically control autoimmunity.919 55,57 Currently, a phase 2/3 (NCT05721573) study in IBM is evaluating ABC008/Ulviprubart, a humanised monoclonal antibody against KLRG1, which selectively depletes highly differentiated T cells. Early data from a pilot study in three IBM patients demonstrated that ABC008 can selectively lower the proportion of circulating KLRG1+CD8+ T cells and T-LGLs.58 Circulating Tregs were not depleted with the drug. Some improvement in functional measures was noted in these patients.
Another challenge in assessing intervention efficacy is the heterogeneity of the disease. IBM presents with varying rates of progression, different symptom profiles (eg, dysphagia, upper or lower limb predominance, asymmetry) and comorbidities, especially given the advanced age of most patients.23 49 59,62 Other factors, such as fatigue, social support, and exercise type, intensity and duration, must be optimised to minimise their influence on outcomes. Although challenging, controlling these variables will facilitate more reliable outcome assessments in future studies.
Meta-analyses of serious adverse events suggest that most pharmacological interventions were well tolerated, with the exception of a slightly higher adverse event rate associated with sirolimus. Generally, adverse events were mild, and discontinuation due to serious side effects ranged from 0%3435 37 39,42 to 33%33 of patients. Two studies lacked sufficient information on serious adverse events,38 43 while rates were reported as 18% in two studies,5 6 7%36 in one, and 6%8 in another. Immunosuppressive treatments were associated with a higher incidence of adverse effects, whereas IVIg studies reported fewer adverse events.
Our study has limitations. It is important to note that this SR focused on RCTs, limiting the scope to high-level evidence and excluding observational studies. Moreover, only 2 of the 14 RCTs were included in meta-analyses due to the small number of trials, lack of sufficient data and small sample sizes.
In conclusion, this review summarises existing evidence on the efficacy and safety of pharmacological interventions for IBM. While most interventions were not effective, they had favourable safety profiles. To advance treatment options, optimising outcome measures, agreeing on a core outcome set and follow-up duration, and accounting for disease-specific and individual factors are crucial. The development of new treatments, alongside advances in understanding IBM pathogenesis, is vital. Further studies are necessary to identify effective treatments for IBM.
supplementary material
Acknowledgements
This paper will contribute towards a Post Doctoral Program for EJFS. This paper was presented as an abstract at EULAR 2024 (Santos E, et al. POS1314: Efficacy of pharmacological treatment in inclusion body myositis: a systematic review. Annals of the Rheumatic Diseases 2024;83:633).
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Rose MR, Jones K, Leong K, et al. Treatment for inclusion body myositis. Cochrane Database Syst Rev. 2015;7:CD001555. doi: 10.1002/14651858.CD001555.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sangha G, Yao B, Lunn D, et al. Longitudinal observational study investigating outcome measures for clinical trials in inclusion body myositis. J Neurol Neurosurg Psychiatry. 2021:jnnp-2020-325141. doi: 10.1136/jnnp-2020-325141. [DOI] [PubMed] [Google Scholar]
- 3.Machado PM, Ahmed M, Brady S, et al. Ongoing developments in sporadic inclusion body myositis. Curr Rheumatol Rep. 2014;16:477. doi: 10.1007/s11926-014-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly CM, Gupta L, Fujimoto M, et al. Idiopathic inflammatory myopathies: current insights and future frontiers. Lancet Rheumatol. 2024;6:e115–27. doi: 10.1016/S2665-9913(23)00322-3. [DOI] [PubMed] [Google Scholar]
- 5.Benveniste O, Hogrel J-Y, Belin L, et al. Sirolimus for treatment of patients with inclusion body myositis: a randomised, double-blind, placebo-controlled, proof-of-concept, phase 2b trial. Lancet Rheumatol. 2021;3:e40–8. doi: 10.1016/S2665-9913(20)30280-0. [DOI] [PubMed] [Google Scholar]
- 6.Machado PM, McDermott MP, Blaettler T, et al. Safety and efficacy of arimoclomol for inclusion body myositis: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2023;22:900–11. doi: 10.1016/S1474-4422(23)00275-2. [DOI] [PubMed] [Google Scholar]
- 7.Benveniste O, Guiguet M, Freebody J, et al. Long-term observational study of sporadic inclusion body myositis. Brain (Bacau) 2011;134:3176–84. doi: 10.1093/brain/awr213. [DOI] [PubMed] [Google Scholar]
- 8.Hanna MG, Badrising UA, Benveniste O, et al. Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): a randomised, double-blind, placebo-controlled phase 2b trial. Lancet Neurol. 2019;18:834–44. doi: 10.1016/S1474-4422(19)30200-5. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg SA. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol. 2019;15:257–72. doi: 10.1038/s41584-019-0186-x. [DOI] [PubMed] [Google Scholar]
- 10.Callan A, Capkun G, Vasanthaprasad V, et al. A Systematic Review and Meta-Analysis of Prevalence Studies of Sporadic Inclusion Body Myositis. J Neuromuscul Dis. 2017;4:127–37. doi: 10.3233/JND-160198. [DOI] [PubMed] [Google Scholar]
- 11.Shelly S, Mielke MM, Mandrekar J, et al. Epidemiology and Natural History of Inclusion Body Myositis: A 40-Year Population-Based Study. Neurology (ECronicon) 2021;96:e2653–61. doi: 10.1212/WNL.0000000000012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keshishian A, Greenberg SA, Agashivala N, et al. Health care costs and comorbidities for patients with inclusion body myositis. Curr Med Res Opin. 2018;34:1679–85. doi: 10.1080/03007995.2018.1486294. [DOI] [PubMed] [Google Scholar]
- 13.McLeish E, Slater N, Sooda A, et al. Inclusion body myositis: The interplay between ageing, muscle degeneration and autoimmunity. Best Pract Res Clin Rheumatol. 2022;36:101761. doi: 10.1016/j.berh.2022.101761. [DOI] [PubMed] [Google Scholar]
- 14.Naddaf E, Barohn RJ, Dimachkie MM. Inclusion Body Myositis: Update on Pathogenesis and Treatment. Neurotherapeutics . 2018;15:995–1005. doi: 10.1007/s13311-018-0658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothwell S, Cooper RG, Lundberg IE, et al. Immune-Array Analysis in Sporadic Inclusion Body Myositis Reveals HLA-DRB1 Amino Acid Heterogeneity Across the Myositis Spectrum. Arthritis Rheumatol . 2017;69:1090–9. doi: 10.1002/art.40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larman HB, Salajegheh M, Nazareno R, et al. Cytosolic 5’-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol. 2013;73:408–18. doi: 10.1002/ana.23840. [DOI] [PubMed] [Google Scholar]
- 17.Pluk H, van Hoeve BJA, van Dooren SHJ, et al. Autoantibodies to cytosolic 5’-nucleotidase 1A in inclusion body myositis. Ann Neurol. 2013;73:397–407. doi: 10.1002/ana.23822. [DOI] [PubMed] [Google Scholar]
- 18.Salam S, Dimachkie MM, Hanna MG, et al. Diagnostic and prognostic value of anti-cN1A antibodies in inclusion body myositis. Clin Exp Rheumatol. 2022;40:384–93. doi: 10.55563/clinexprheumatol/r625rm. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg SA, Pinkus JL, Kong SW, et al. Highly differentiated cytotoxic T cells in inclusion body myositis. Brain (Bacau) 2019;142:2590–604. doi: 10.1093/brain/awz207. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). In: Cochrane, Ed. Cochrane; 2019. [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones K, Pitceathly RDS, Rose MR, et al. Interventions for dysphagia in long-term, progressive muscle disease. Cochrane Database Syst Rev. 2016;2:CD004303. doi: 10.1002/14651858.CD004303.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griggs RC, Askanas V, DiMauro S, et al. Inclusion body myositis and myopathies. Ann Neurol. 1995;38:705–13. doi: 10.1002/ana.410380504. [DOI] [PubMed] [Google Scholar]
- 24.Benveniste O, Hilton-Jones D. International Workshop on Inclusion Body Myositis held at the Institute of Myology, Paris, on 29 May 2009. Neuromuscul Disord . 2010;20:414–21. doi: 10.1016/j.nmd.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Hilton-Jones D, Miller A, Parton M, et al. Inclusion body myositis: MRC Centre for Neuromuscular Diseases, IBM workshop, London, 13 June 2008. Neuromuscul Disord. 2010;20:142–7. doi: 10.1016/j.nmd.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Rose MR, ENMC IBM Working Group 188th ENMC International Workshop: Inclusion Body Myositis, 2-4 December 2011, Naarden, The Netherlands. Neuromuscul Disord. 2013;23:1044–55. doi: 10.1016/j.nmd.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 27.European Union Directive 2001/83/EC of the European parliament and of the council of 6 November 2001 on the community code relating to medicinal products for human use. Official journal of the European communities no l-311/67 of 28 November 2001. 2001
- 28.OCEBM Levels of Evidence Working Group The oxford levels of evidence. Oxford centre for evidence-based medicine (updated in 2012) 2009. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence Available.
- 29.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos E, Cardoso D, Apóstolo J. How to measure and explore heterogeneity in a meta-analysis: Key methodological strategies. Rev Enferm Ref. 2022;6:e21077. doi: 10.12707/RV21077. [DOI] [Google Scholar]
- 31.Tufanaru C, Munn Z, Stephenson M, et al. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13:196–207. doi: 10.1097/XEB.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 32.Tufanaru C, Munn Z, Aromataris E, et al. In: Joanna Briggs Institute Reviewer’s Manual. Aromataris E, Munn Z, editors. The Joanna Briggs Institute; 2017. Chapter 3: systematic reviews of effectiveness. [Google Scholar]
- 33.Badrising UA, Maat-Schieman MLC, Ferrari MD, et al. Comparison of weakness progression in inclusion body myositis during treatment with methotrexate or placebo. Ann Neurol. 2002;51:369–72. doi: 10.1002/ana.10121. [DOI] [PubMed] [Google Scholar]
- 34.Amato AA, Sivakumar K, Goyal N, et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology (ECronicon) 2014;83:2239–46. doi: 10.1212/WNL.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalakas MC, Koffman B, Fujii M, et al. A controlled study of intravenous immunoglobulin combined with prednisone in the treatment of IBM. Neurology (ECronicon) 2001;56:323–7. doi: 10.1212/wnl.56.3.323. [DOI] [PubMed] [Google Scholar]
- 36.The Muscle Study Group Randomized pilot trial of high-dose βINF-1a in patients with inclusion body myositis. Neurology (ECronicon) 2004;63:718–20. doi: 10.1212/01.WNL.0000134675.98525.79. [DOI] [PubMed] [Google Scholar]
- 37.The Muscle Study Group* Randomized pilot trial of βINF1a (Avonex) in patients with inclusion body myositis. Neurol (ECronicon) 2001;57:1566–70. doi: 10.1212/WNL.57.9.1566. [DOI] [PubMed] [Google Scholar]
- 38.Rutkove SB, Parker RA, Nardin RA, et al. A pilot randomized trial of oxandrolone in inclusion body myositis. Neurology (ECronicon) 2002;58:1081–7. doi: 10.1212/wnl.58.7.1081. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed M, Machado PM, Miller A, et al. Targeting protein homeostasis in sporadic inclusion body myositis. Sci Transl Med. 2016;8:331ra41. doi: 10.1126/scitranslmed.aad4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalakas MC, Sonies B, Dambrosia J, et al. Treatment of inclusion-body myositis with IVIg: a double-blind, placebo-controlled study. Neurology (ECronicon) 1997;48:712–6. doi: 10.1212/wnl.48.3.712. [DOI] [PubMed] [Google Scholar]
- 41.Walter MC, Lochmüller H, Toepfer M, et al. High-dose immunoglobulin therapy in sporadic inclusion body myositis: a double-blind, placebo-controlled study. J Neurol. 2000;247:22–8. doi: 10.1007/s004150050005. [DOI] [PubMed] [Google Scholar]
- 42.Lindberg C, Trysberg E, Tarkowski A, et al. Anti-T-lymphocyte globulin treatment in inclusion body myositis: a randomized pilot study. Neurology (ECronicon) 2003;61:260–2. doi: 10.1212/01.wnl.0000071852.27182.c7. [DOI] [PubMed] [Google Scholar]
- 43.Leff RL, Miller FW, Hicks J, et al. The treatment of inclusion body myositis: a retrospective review and a randomized, prospective trial of immunosuppressive therapy. Medicine (Baltimore) 1993;72:225–35. doi: 10.1097/00005792-199307000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Roy B, Lucchini M, Lilleker JB, et al. Current status of clinical outcome measures in inclusion body myositis: a systematised review. Clin Exp Rheumatol. 2023;41:370–8. doi: 10.55563/clinexprheumatol/ifacv3. [DOI] [PubMed] [Google Scholar]
- 45.Perez-Rosendahl M, Mozaffar T. Inclusion body myositis: evolving concepts. Curr Opin Neurol. 2022;35:604–10. doi: 10.1097/WCO.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alfano LN, Focht Garand KL, Malandraki GA, et al. Measuring change in inclusion body myositis: clinical assessments versus imaging. Clin Exp Rheumatol. 2022;40:404–13. doi: 10.55563/clinexprheumatol/0q2voe. [DOI] [PubMed] [Google Scholar]
- 47.Salam S, Symonds T, Doll H, et al. Measurement properties of the Inclusion Body Myositis Functional Rating Scale. J Neurol Neurosurg Psychiatry. 2024:jnnp-2024-333617. doi: 10.1136/jnnp-2024-333617. [DOI] [PubMed] [Google Scholar]
- 48.Symonds T, Randall J, Lloyd-Price L, et al. Study to Assess Content Validity and Interrater and Intrarater Reliability of the Inclusion Body Myositis Functional Rating Scale. Neurol Clin Pract. 2023;13:e200168. doi: 10.1212/CPJ.0000000000200168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lilleker JB, Naddaf E, Saris CGJ, et al. 272nd ENMC international workshop: 10 Years of progress - revision of the ENMC 2013 diagnostic criteria for inclusion body myositis and clinical trial readiness. 16–18 June 2023, Hoofddorp, The Netherlands. Neuromuscul Disord. 2024;37:36–51. doi: 10.1016/j.nmd.2024.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Anderson NC, Lloyd TE. Inclusion body myositis: an update. Curr Opin Rheumatol. 2025;37:80–5. doi: 10.1097/BOR.0000000000001060. [DOI] [PubMed] [Google Scholar]
- 51.Reyngoudt H, Baudin P-Y, Caldas de Almeida Araújo E, et al. Effect of sirolimus on muscle in inclusion body myositis observed with magnetic resonance imaging and spectroscopy. J Cachexia Sarcopenia Muscle. 2024;15:1108–20. doi: 10.1002/jcsm.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurent D, Riek J, Sinclair CDJ, et al. Longitudinal Changes in MRI Muscle Morphometry and Composition in People With Inclusion Body Myositis. Neurology (ECronicon) 2022;99:e865–76. doi: 10.1212/WNL.0000000000200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlaffke L, Rehmann R, Froeling M, et al. Quantitative muscle MRI in sporadic inclusion body myositis (sIBM): A prospective cohort study. J Neuromuscul Dis. 2024;11:997–1009. doi: 10.3233/JND-240053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allameen NA, Salam S, Reddy V, et al. Inclusion body myositis and immunosenescence: current evidence and future perspectives. Rheumatol (Oxford) 2024:keae614. doi: 10.1093/rheumatology/keae614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 56.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 57.Henson SM, Akbar AN. KLRG1--more than a marker for T cell senescence. Age (Dordr) 2009;31:285–91. doi: 10.1007/s11357-009-9100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goel N, Needham M, Soler-Ferran D, et al. POS1342 DEPLETION OF KLRG1+ T CELLS IN A FIRST-IN-HUMAN CLINICAL TRIAL OF ABC008 IN INCLUSION BODY MYOSITIS (IBM) Ann Rheum Dis. 2022;81:1008–9. doi: 10.1136/annrheumdis-2022-eular.2141. [DOI] [Google Scholar]
- 59.Salam S, Morrow JM, Howard R, et al. Two emerging phenotypes of atypical inclusion body myositis: illustrative cases. Clin Exp Rheumatol. 2023;41:340–7. doi: 10.55563/clinexprheumatol/jq7zxd. [DOI] [PubMed] [Google Scholar]
- 60.Nagy S, Khan A, Machado PM, et al. Inclusion body myositis: from genetics to clinical trials. J Neurol. 2023;270:1787–97. doi: 10.1007/s00415-022-11459-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machado PM, Dimachkie MM, Barohn RJ. Sporadic inclusion body myositis: new insights and potential therapy. Curr Opin Neurol. 2014;27:591–8. doi: 10.1097/WCO.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roy B, Dimachkie MM, Naddaf E. Phenotypic spectrum of inclusion body myositis. Clin Exp Rheumatol. 2024;42:445–53. doi: 10.55563/clinexprheumatol/fhrx3q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.