Abstract
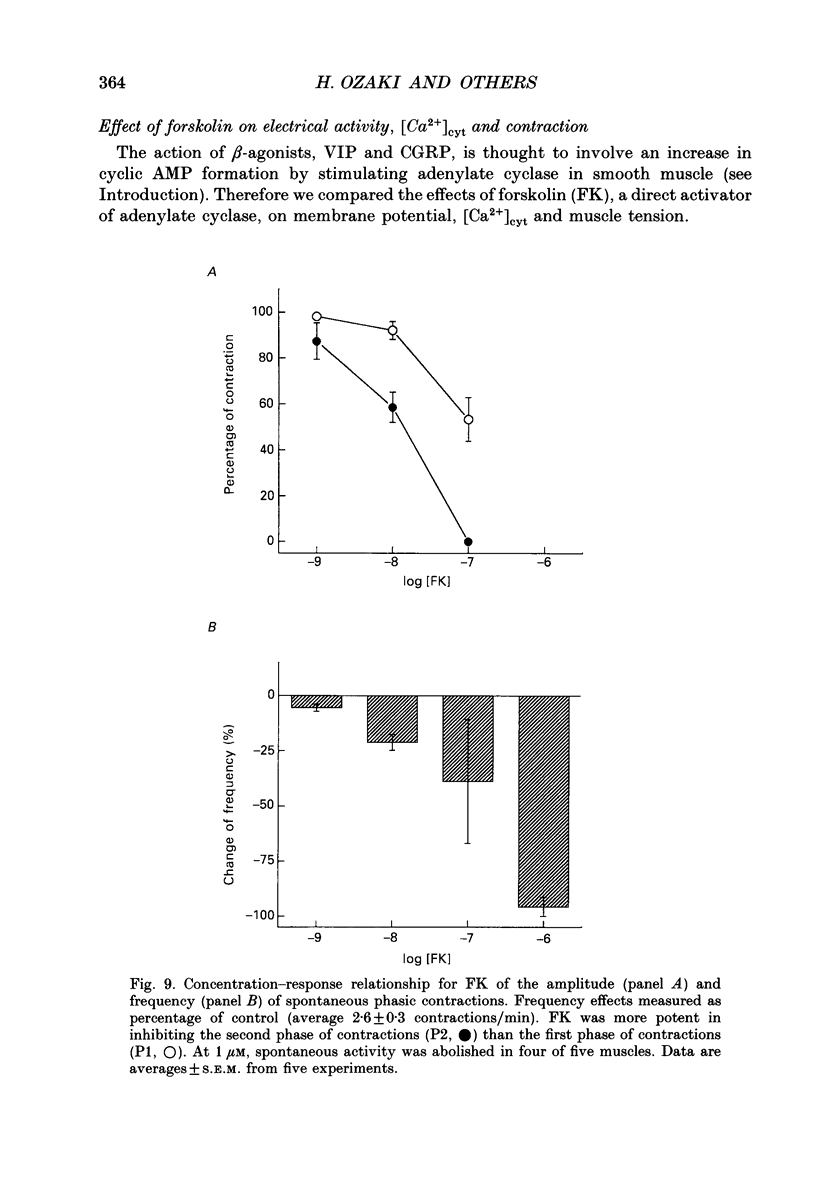
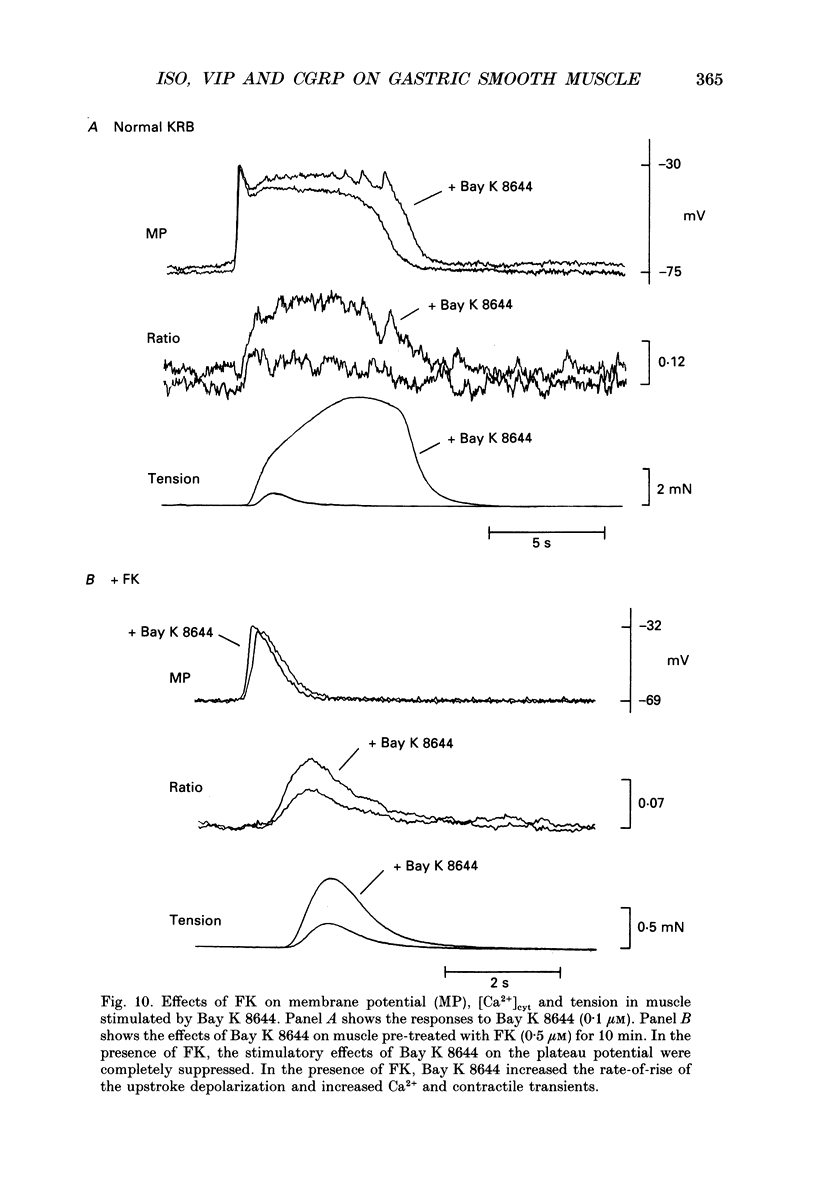
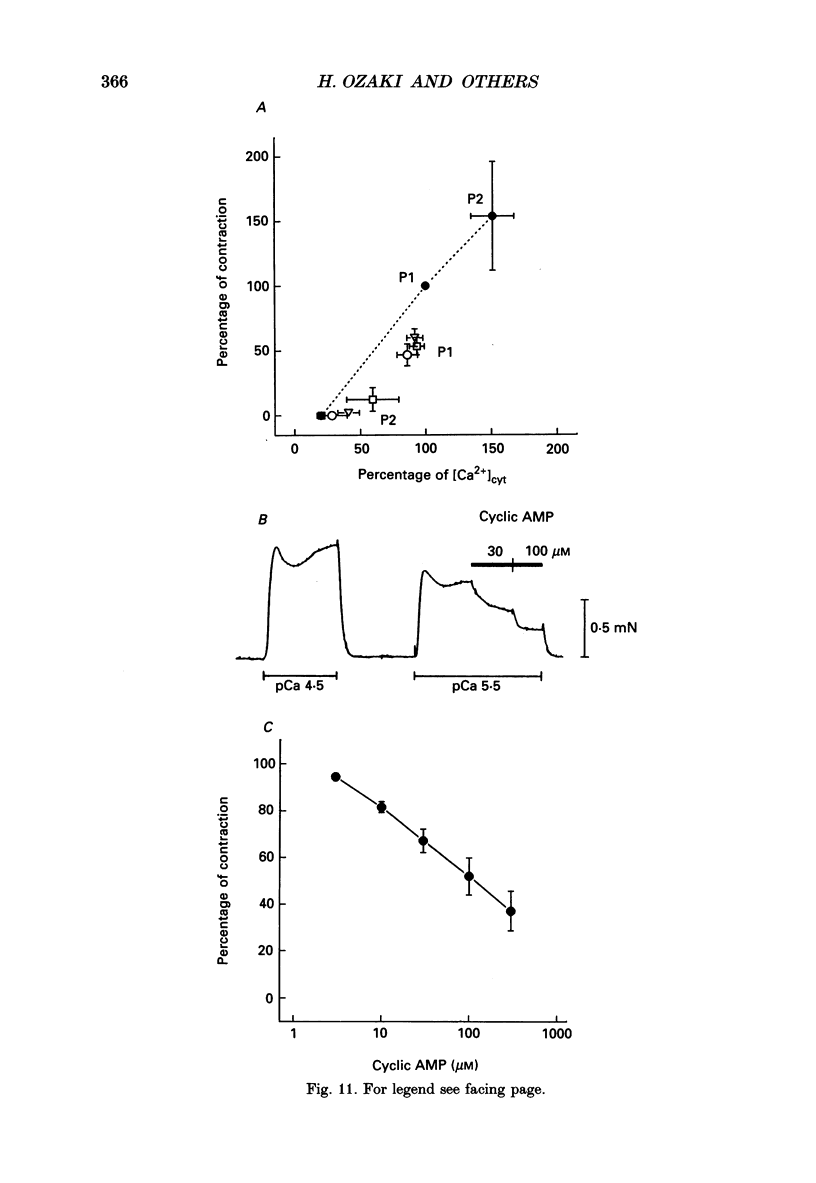
1. Agonists known to increase cyclic AMP levels in gastrointestinal smooth muscles were studied in isolated circular muscles of the canine antrum to investigate the mechanisms of the inhibitory effects of these agents. 2. Muscles were electrically active, generating typical slow wave activity. Cytosolic Ca2+ ([Ca2+]cyt; measured by Indo-1 fluorescence) and tension increased in response to slow waves. 3. Stimulation by isoprenaline (via beta 2-receptors) or forskolin, in the presence or absence of acetylcholine, inhibited the plateau phase and reduced phasic [Ca2+]cyt and contractile responses. 4. Vasoactive intestinal peptide (VIP) and calcitonin gene-related peptide (CGRP), had similar effects to isoprenaline and forskolin. 5. Increases in the plateau phase of slow waves and the associated increases in [Ca2+]cyt and tension caused by direct activation of voltage-dependent Ca2+ channels by Bay K 8644 (0.1 microM) were also reduced by forskolin. 6. Isoprenaline and forskolin induced negative chronotropic effects, but VIP increased frequency. 7. At a given level of [Ca2+]cyt, contractions were greater under control conditions than in the presence of isoprenaline, VIP and CGRP, suggesting that part of the inhibition produced by these agents may be due to decreased Ca2+ sensitivity of the contractile apparatus. 8. Experiments performed on alpha-toxin-permeabilized muscles confirmed that cyclic AMP-dependent effects involve reduced Ca2+ sensitivity of the contractile apparatus. Addition of cyclic AMP (3-300 microM) caused a reduction in Ca(2+)-induced contraction at a constant level of Ca2+ (pCa 5.5). 9. These results suggest that increased cyclic AMP and probably subsequent activation of protein kinase A: (i) decrease [Ca2+]cyt and contraction by an inhibition of Ca2+ influx during slow waves, and (ii) decrease the sensitivity of the contractile apparatus to [Ca2+]cyt. The membrane effects might occur directly by inhibition of Ca2+ channels or indirectly by increasing the open probability of K+ channels which would tend to cause premature repolarization of slow waves.
Full text
PDF


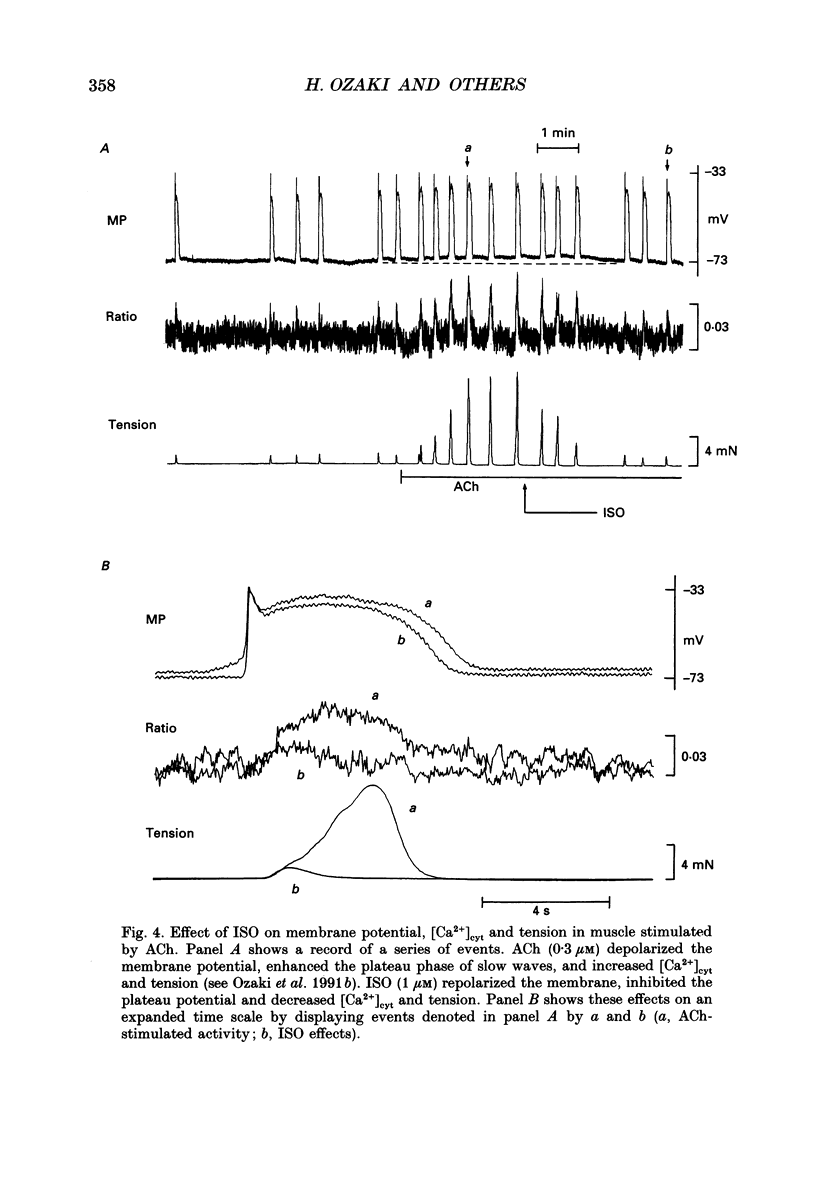
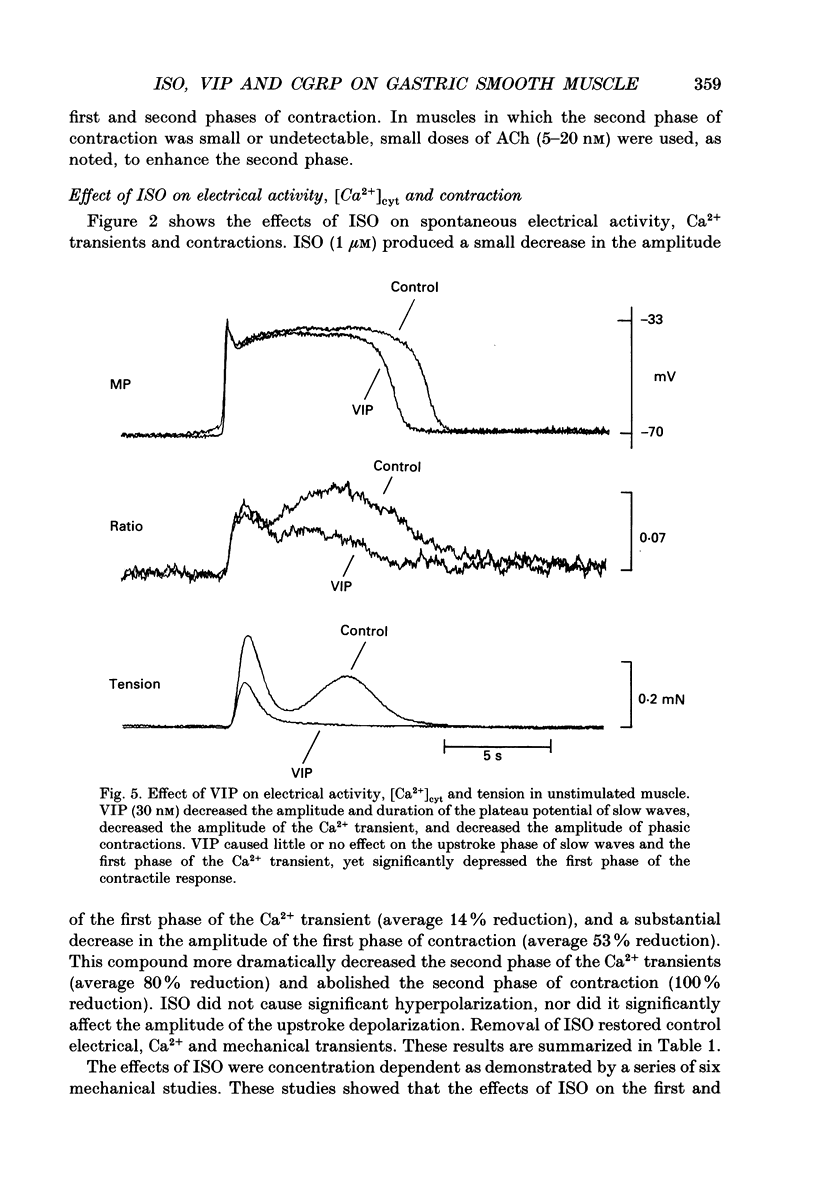
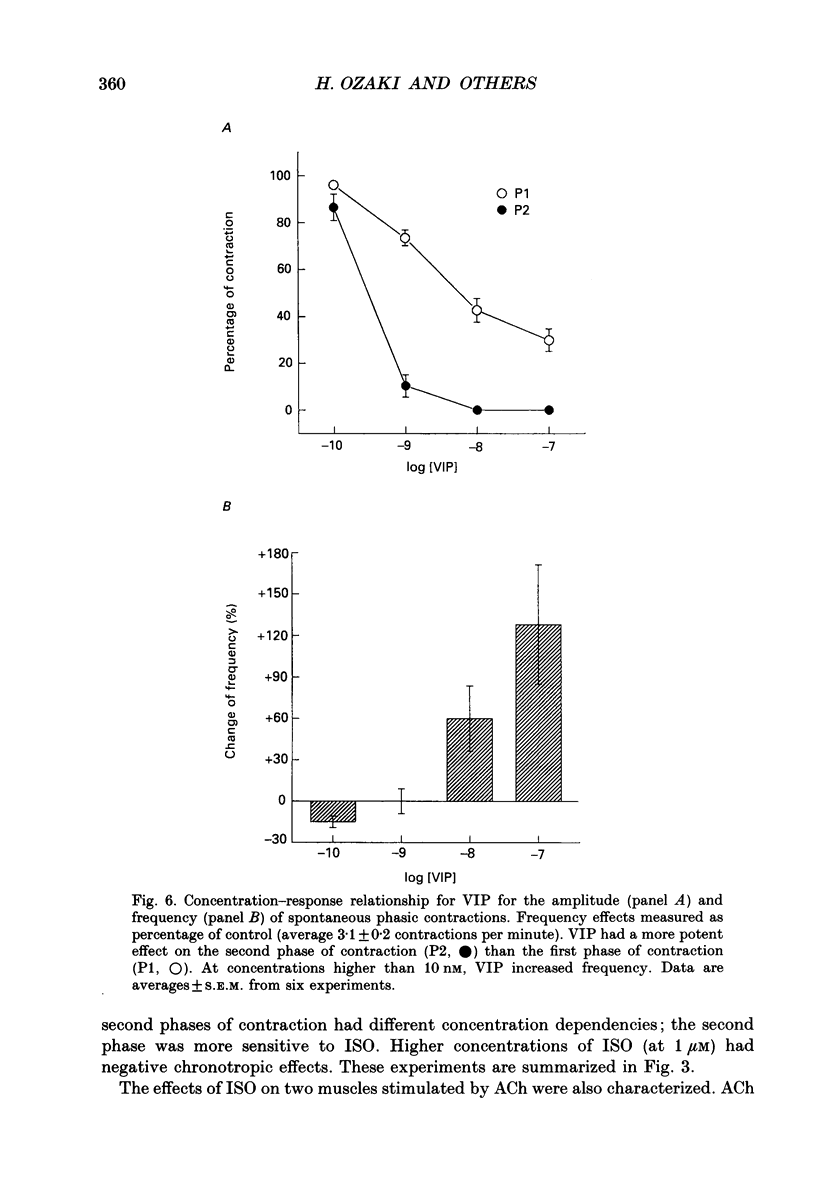
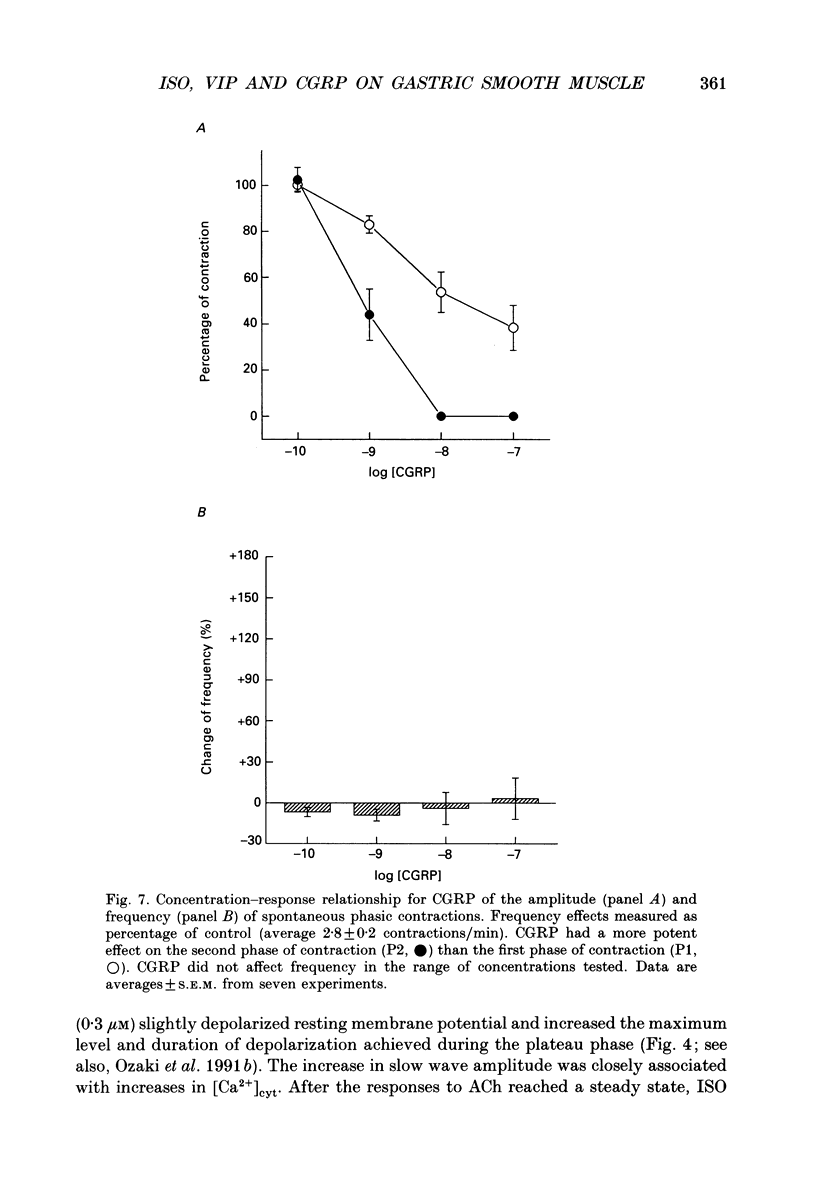







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe A., Karaki H. Effect of forskolin on cytosolic Ca++ level and contraction in vascular smooth muscle. J Pharmacol Exp Ther. 1989 Jun;249(3):895–900. [PubMed] [Google Scholar]
- Barthó L., Lembeck F., Holzer P. Calcitonin gene-related peptide is a potent relaxant of intestinal muscle. Eur J Pharmacol. 1987 Mar 31;135(3):449–451. doi: 10.1016/0014-2999(87)90699-6. [DOI] [PubMed] [Google Scholar]
- Bauer A. J., Reed J. B., Sanders K. M. Slow wave heterogeneity within the circular muscle of the canine gastric antrum. J Physiol. 1985 Sep;366:221–232. doi: 10.1113/jphysiol.1985.sp015793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar K. N., Makhlouf G. M. Relaxation of isolated gastric smooth muscle cells by vasoactive intestinal peptide. Science. 1982 Apr 30;216(4545):531–533. doi: 10.1126/science.6176025. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Catecholamine action on smooth muscle. Pharmacol Rev. 1987 Mar;39(1):49–96. [PubMed] [Google Scholar]
- Carl A., Kenyon J. L., Uemura D., Fusetani N., Sanders K. M. Regulation of Ca(2+)-activated K+ channels by protein kinase A and phosphatase inhibitors. Am J Physiol. 1991 Aug;261(2 Pt 1):C387–C392. doi: 10.1152/ajpcell.1991.261.2.C387. [DOI] [PubMed] [Google Scholar]
- Carl A., McHale N. G., Publicover N. G., Sanders K. M. Participation of Ca2(+)-activated K+ channels in electrical activity of canine gastric smooth muscle. J Physiol. 1990 Oct;429:205–221. doi: 10.1113/jphysiol.1990.sp018252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J., Haglund U., Jodal M., Lundgren O., Olbe L., de Muckadell O. B. Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiological implications. J Physiol. 1978 Nov;284:291–305. doi: 10.1113/jphysiol.1978.sp012541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Ito S., Kurokawa A., Ohga A., Ohta T., Sawabe K. Mechanical, electrical and cyclic nucleotide responses to peptide VIP and inhibitory nerve stimulation in rat stomach. J Physiol. 1990 Nov;430:337–353. doi: 10.1113/jphysiol.1990.sp018294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H. Ca2+ localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci. 1989 Aug;10(8):320–325. doi: 10.1016/0165-6147(89)90066-7. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Takasaki K., Saito A., Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988 Sep 8;335(6186):164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Lefebvre R. A., Verplanken P. A., Bogaert M. G. Pharmacological characterization of the postjunctional beta-adrenoceptors in the rat gastric fundus. Eur J Pharmacol. 1984 Oct 30;106(1):1–9. doi: 10.1016/0014-2999(84)90671-x. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Fisher-Simpson V. A comparison of the effects of forskolin and nitroprusside on cyclic nucleotides and relaxation in the rat aorta. Eur J Pharmacol. 1984 May 18;101(1-2):17–27. doi: 10.1016/0014-2999(84)90026-8. [DOI] [PubMed] [Google Scholar]
- Maton P. N., Sutliff V. E., Zhou Z. C., Collins S. M., Gardner J. D., Jensen R. T. Characterization of receptors for calcitonin gene-related peptide on gastric smooth muscle cells. Am J Physiol. 1988 Jun;254(6 Pt 1):G789–G794. doi: 10.1152/ajpgi.1988.254.6.G789. [DOI] [PubMed] [Google Scholar]
- Morgan K. G., Muir T. C., Szurszewski J. H. The electrical basis for contraction and relaxation in canine fundal smooth muscle. J Physiol. 1981 Feb;311:475–488. doi: 10.1113/jphysiol.1981.sp013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K. G., Schmalz P. F., Szurszewski J. H. The inhibitory effects of vasoactive intestinal polypeptide on the mechanical and electrical activity of canine antral smooth muscle. J Physiol. 1978 Sep;282:437–450. doi: 10.1113/jphysiol.1978.sp012474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K. G., Szurszewski J. H. Mechanisms of phasic and tonic actions of pentagastrin on canine gastric smooth muscle. J Physiol. 1980 Apr;301:229–242. doi: 10.1113/jphysiol.1980.sp013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. J., Baer H. P. Relaxant effects of forskolin in smooth muscle. Role of cyclic AMP. Naunyn Schmiedebergs Arch Pharmacol. 1983 Feb;322(1):78–82. doi: 10.1007/BF00649356. [DOI] [PubMed] [Google Scholar]
- Nishimura J., van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989 Sep 15;163(2):929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Gerthoffer W. T., Publicover N. G., Fusetani N., Sanders K. M. Time-dependent changes in Ca2+ sensitivity during phasic contraction of canine antral smooth muscle. J Physiol. 1991;440:207–224. doi: 10.1113/jphysiol.1991.sp018704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Kasai H., Hori M., Sato K., Ishihara H., Karaki H. Direct inhibition of chicken gizzard smooth muscle contractile apparatus by caffeine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Mar;341(3):262–267. doi: 10.1007/BF00169741. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Kwon S. C., Tajimi M., Karaki H. Changes in cytosolic CA2+ and contraction induced by various stimulants and relaxants in canine tracheal smooth muscle. Pflugers Arch. 1990 Jun;416(4):351–359. doi: 10.1007/BF00370740. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Satoh T., Karaki H., Ishida Y. Regulation of metabolism and contraction by cytoplasmic calcium in the intestinal smooth muscle. J Biol Chem. 1988 Oct 5;263(28):14074–14079. [PubMed] [Google Scholar]
- Ozaki H., Stevens R. J., Blondfield D. P., Publicover N. G., Sanders K. M. Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles. Am J Physiol. 1991 May;260(5 Pt 1):C917–C925. doi: 10.1152/ajpcell.1991.260.5.C917. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Burke E. P., Carl A., Cole W. C., Langton P., Ward S. Mechanism of electrical rhythmicity in colonic smooth muscle: an hypothesis. Prog Clin Biol Res. 1990;327:307–321. [PubMed] [Google Scholar]
- Somlyo A. P., Himpens B. Cell calcium and its regulation in smooth muscle. FASEB J. 1989 Sep;3(11):2266–2276. doi: 10.1096/fasebj.3.11.2506092. [DOI] [PubMed] [Google Scholar]
- Sternini C., Reeve J. R., Jr, Brecha N. Distribution and characterization of calcitonin gene-related peptide immunoreactivity in the digestive system of normal and capsaicin-treated rats. Gastroenterology. 1987 Oct;93(4):852–862. doi: 10.1016/0016-5085(87)90450-1. [DOI] [PubMed] [Google Scholar]
- Stiles G. L., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptors: biochemical mechanisms of physiological regulation. Physiol Rev. 1984 Apr;64(2):661–743. doi: 10.1152/physrev.1984.64.2.661. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Hescheler J. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Annu Rev Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- Vogalis F., Publicover N. G., Hume J. R., Sanders K. M. Relationship between calcium current and cytosolic calcium in canine gastric smooth muscle cells. Am J Physiol. 1991 May;260(5 Pt 1):C1012–C1018. doi: 10.1152/ajpcell.1991.260.5.C1012. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Vogalis F., Blondfield D. P., Ozaki H., Fusetani N., Uemura D., Publicover N. G., Sanders K. M. Inhibition of electrical slow waves and Ca2+ currents of gastric and colonic smooth muscle by phosphatase inhibitors. Am J Physiol. 1991 Jul;261(1 Pt 1):C64–C70. doi: 10.1152/ajpcell.1991.261.1.C64. [DOI] [PubMed] [Google Scholar]
- Yagi S., Becker P. L., Fay F. S. Relationship between force and Ca2+ concentration in smooth muscle as revealed by measurements on single cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4109–4113. doi: 10.1073/pnas.85.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sharkawy T. Y., Szurszewski J. H. Modulation of canine antral circular smooth muscle by acetylcholine, noradrenaline and pentagastrin. J Physiol. 1978 Jun;279:309–320. doi: 10.1113/jphysiol.1978.sp012346. [DOI] [PMC free article] [PubMed] [Google Scholar]


