Abstract
1. In isolated mouse diaphragm, nerve stimulation in the presence of Sr2+ evokes phasic quantal transmitter release (endplate potentials, EPPs) with the same time course as in the presence of Ca2+. 2. Brief tetanic trains of nerve stimuli in the presence of Sr2+ cause an increase in quantal content of EPPs accompanied by an increase in the frequency of miniature EPPs (MEPPs); the latter persists as a 'tail' that subsides within about a second. Pseudo-random stimulation sequences were used to characterize these changes. 3. The fourth root of MEPP frequency during or after stimulation rose and fell in accordance with first order kinetics with the same time constants for rising and falling phases, in agreement with a 'residual ion' model in which (a) each nerve impulse causes the same entry of Sr2+ into the nerve terminal, (b) transmitter release (MEPP frequency) is proportional to the fourth power of [Sr2+] at release sites, and (c) Sr2+ removal is a first order process with a time constant of about 250 ms. 4. After exposure to bis (O-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid, acetoxymethyl ester form (BAPTA AM), 'Sr2+ tails' of MEPP frequency were reduced in magnitude and prolonged. 5. During stimulation trains, growth of phasic transmitter release rates (and quantal content of EPPs) were related to growth of MEPP frequency in almost exact agreement with a residual ion model, in which 'phasic' release (EPPs) and MEPP frequency are governed by the same equation, with the same parameters, and without any effect of depolarization per se to affect phasic release. 6. Prolonged (1 s) nerve terminal depolarizations in the presence of Sr2+ produce increased MEPP frequency with a time course corresponding to a model in which depolarization per se has little or no effect to increase transmitter release. 7. It was concluded that in the presence of Sr2+ the intense 'phasic' acceleration of quantal release induced by nerve impulse manifest in an EPP can be attributed to a transient rise of intracellular [Sr2+] in the vicinity of release sites, while the modulation of 'phasic' release by antecedent nerve impulses can be attributed to residual Sr2+ which is also manifest in a rise in MEPP frequency.
Full text
PDF





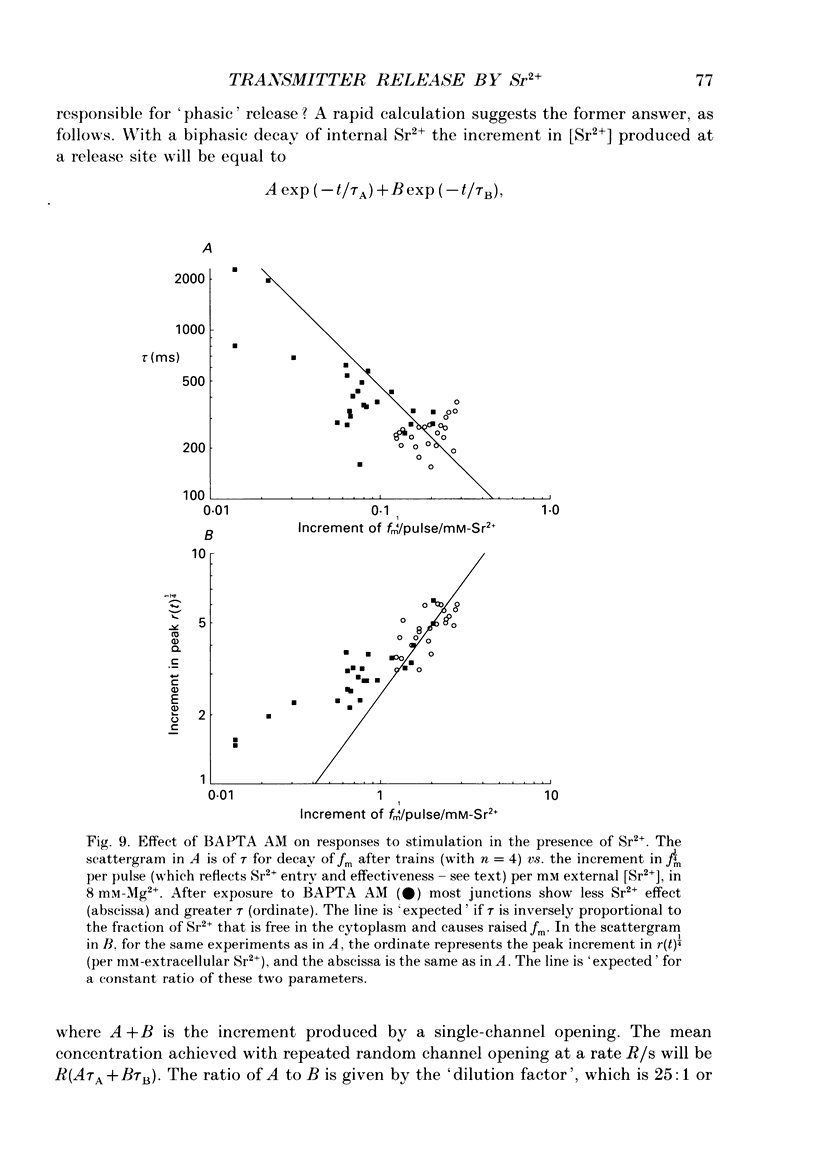
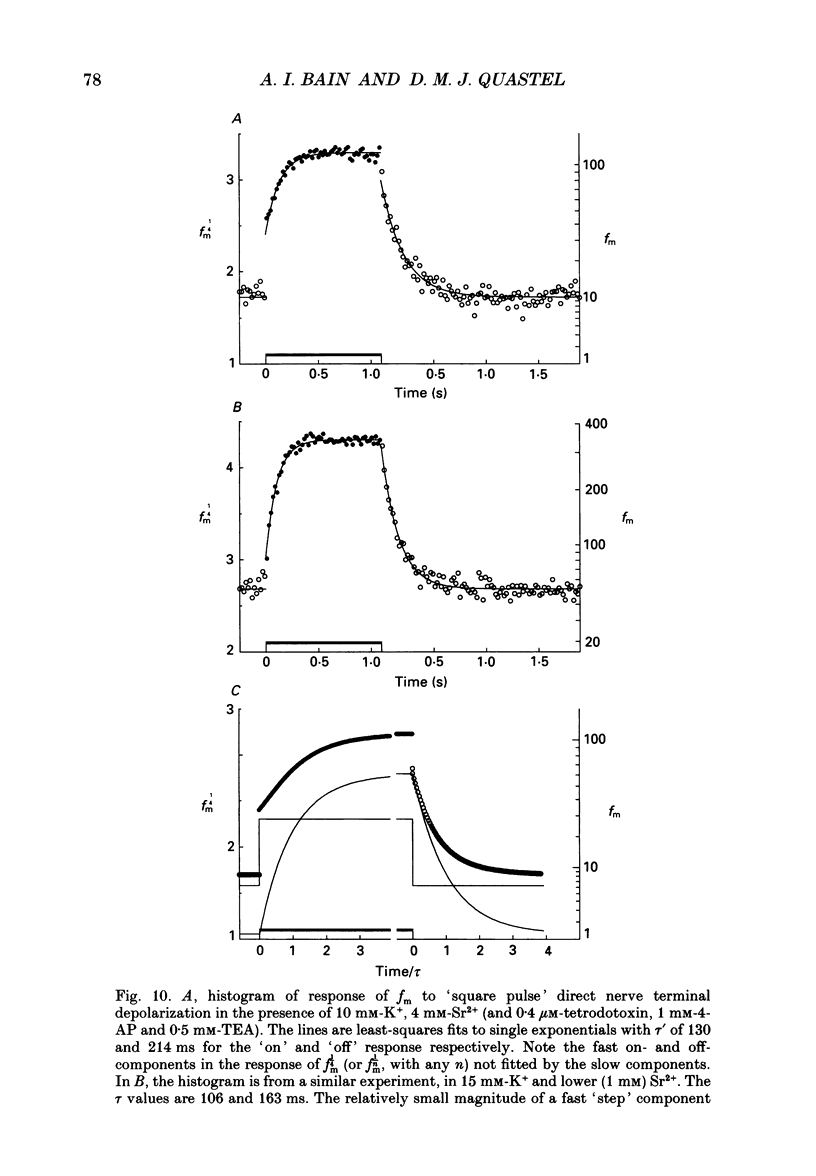









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Baldo G. J., Cohen I. S., Van der Kloot W. Estimating the time course of evoked quantal release at the frog neuromuscular junction using end-plate current latencies. J Physiol. 1986 May;374:503–513. doi: 10.1113/jphysiol.1986.sp016094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. Quantal independence and uniformity of presynaptic release kinetics at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):665–689. doi: 10.1113/jphysiol.1972.sp010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Cumulative and persistent effects of nerve terminal depolarization on transmitter release. J Physiol. 1973 Jan;228(2):407–434. doi: 10.1113/jphysiol.1973.sp010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Biophysical aspects of neuro-muscular transmission. Prog Biophys Biophys Chem. 1956;6:121–170. [PubMed] [Google Scholar]
- Datyner N. B., Gage P. W. Phasic secretion of acetylcholine at a mammalian neuromuscular junction. J Physiol. 1980 Jun;303:299–314. doi: 10.1113/jphysiol.1980.sp013286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W. The effect of methyl, ethyl and n-propyl alcohol on neuromuscular transmission in the rat. J Pharmacol Exp Ther. 1965 Nov;150(2):236–243. [PubMed] [Google Scholar]
- Guan Y. Y., Quastel D. M., Saint D. A. Single Ca2+ entry and transmitter release systems at the neuromuscular synapse. Synapse. 1988;2(5):558–564. doi: 10.1002/syn.890020512. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I. REPETITIVE STIMULATION AT THE MAMMALIAN NEUROMUSCULAR JUNCTION, AND THE MOBILIZATION OF TRANSMITTER. J Physiol. 1963 Dec;169:641–662. doi: 10.1113/jphysiol.1963.sp007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima H., Tanabe N. Calcium-independent increase of transmitter release at frog end-plate by trinitrobenzene sulphonic acid. J Physiol. 1988 Sep;403:135–149. doi: 10.1113/jphysiol.1988.sp017243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon J. G., Saint D. A., Quastel D. M. The actions of dimethyl sulfoxide on neuromuscular transmission. Mol Pharmacol. 1986 Dec;30(6):631–638. [PubMed] [Google Scholar]
- Misler S., Falke L., Martin S. Cation dependence of posttetanic potentiation of neuromuscular transmission. Am J Physiol. 1987 Jan;252(1 Pt 1):C55–C62. doi: 10.1152/ajpcell.1987.252.1.C55. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Zucker R. S. Action potentials must admit calcium to evoke transmitter release. Nature. 1991 Mar 14;350(6314):153–155. doi: 10.1038/350153a0. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. Influx of calcium, strontium, and barium in presynaptic nerve endings. J Gen Physiol. 1982 Jun;79(6):1065–1087. doi: 10.1085/jgp.79.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H., Dudel J., Parnas I. Neurotransmitter release and its facilitation in crayfish. VII. Another voltage dependent process beside Ca entry controls the time course of phasic release. Pflugers Arch. 1986 Feb;406(2):121–130. doi: 10.1007/BF00586672. [DOI] [PubMed] [Google Scholar]
- Parnas I., Parnas H. The 'Ca-voltage' hypothesis for neurotransmitter release. Biophys Chem. 1988 Feb;29(1-2):85–93. doi: 10.1016/0301-4622(88)87027-3. [DOI] [PubMed] [Google Scholar]
- Quastel D. M., Saint D. A. Transmitter release at mouse motor nerve terminals mediated by temporary accumulation of intracellular barium. J Physiol. 1988 Dec;406:55–73. doi: 10.1113/jphysiol.1988.sp017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint D. A., Quastel D. M., Guan Y. Y. Multiple potassium conductances at the mammalian motor nerve terminal. Pflugers Arch. 1987 Nov;410(4-5):408–412. doi: 10.1007/BF00586518. [DOI] [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. Estimating the timing of quantal releases during end-plate currents at the frog neuromuscular junction. J Physiol. 1988 Aug;402:595–603. doi: 10.1113/jphysiol.1988.sp017224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. The kinetics of quantal releases during end-plate currents at the frog neuromuscular junction. J Physiol. 1988 Aug;402:605–626. doi: 10.1113/jphysiol.1988.sp017225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Augmentation and facilitation of transmitter release. A quantitative description at the frog neuromuscular junction. J Gen Physiol. 1982 Oct;80(4):583–611. doi: 10.1085/jgp.80.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Changes in miniature endplate potential frequency during repetitive nerve stimulation in the presence of Ca2+, Ba2+, and Sr2+ at the frog neuromuscular junction. J Gen Physiol. 1981 May;77(5):503–529. doi: 10.1085/jgp.77.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Differential effects of Ba2+, Sr2+, and Ca2+ on stimulation-induced changes in transmitter release at the frog neuromuscular junction. J Gen Physiol. 1980 Aug;76(2):175–211. doi: 10.1085/jgp.76.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


