Abstract
Background
To evaluate the prognostic role of the preoperative pan-immune-inflammation value (PIV) index in patients with oral squamous cell carcinoma (OSCC) after undergoing radical resection and to develop a prognostic prediction model for these patients.
Methods
A large cohort study was conducted between January 2015 and March 2022. Univariate and multivariate Cox regression was used to assess the prognostic value of PIV, and propensity score matching (PSM) analysis was used to adjust for potential confounders. Randomized survival forest (RSF) was used to assess the relative importance of preoperative PIV in prognostic prediction. Finally, a Nomogram model was plotted to predict the prognosis of oral cancer patients.
Results
A total of 779 patients were enrolled and followed up (mean follow-up time 34.14 ± 24.39). High PIV was significantly associated with worse survival in OSCC patients (hazard ratio [HR] = 1.62, 95% confidence interval [CI]: 1.15–2.29, P = 0.006). The same trend was observed in PSM (HR = 1.55,95% CI: 1.03–2.23, P = 0.035). RSF showed that PIV ranked third in the importance ranking of all prognostic factors. The calibration curves indicated that the Nomogram model was superior in predicting the prognostic 1-, 3-, and 5-year survival of oral cancer patients.
Conclusions
PIV is an independent predictor of prognosis in patients with oral squamous cell carcinoma, and a column-line graphical model based on PIV can effectively predict prognosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-025-05477-6.
Keywords: PIV, Oral squamous cell carcinoma, Prognosis, Nomogram
Background
Oral cancer is a prevalent type of head and neck tumor that primarily originates from the oral cavity, commonly involving the lips, tongue, palate, mucous membranes of the cheeks, gums, and the floor of the mouth, among other sites [1]. Among oral cancers, the most predominant type is oral squamous cell carcinoma (OSCC), which accounts for about 90% of oral cancers [2]. In a retrospective study [3], the histopathological (HP) subtype of oral cancer was oral squamous carcinoma in 91.5% of cases and the study found that the tumor staging, histological differentiation and local-regional metastasis were the main factors affecting the prognosis of oral cancer, however, most of the cases were diagnosed at advanced stages with little chance of survival, the study reveals the importance of preventive measures. Although following surgical treatment, more than half of the oral cancer patients recurred within two years [4], with a five-year survival rate of approximately 60% [5]. This constitutes a serious global health issue, emphasizing the importance of selecting biomarkers that accurately predict prognosis for patients with OSCC undergoing surgical treatment.
Immunoinflammatory biomarkers (IIBs) play an essential role in cancer prognosis by reflecting the balance between the host’s immune and inflammatory status. They have become valuable prognostic tools in OSCC due to their high availability and cost-effectiveness [6]. Several studies have evaluated the prognostic role of IIB in cancer, and IIB, either alone or in combination with other blood parameters, has been reported to be an important prognostic biomarker for a variety of cancers, including neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), monocyte-lymphocyte ratio (LMR), and systemic immuno-inflammatory index (SII) [4, 7–9]. In the study performed by Roi et al. [10], their results report that SII is a promising new indicator that can predict the prognosis of patients with severe odontogenic infections. Existing pathogenic bacteria that are localized in the tooth structures or periodontal tissues that predispose to serious infections such as odontogenic cervicofacial cellulitis, it is also inextricably linked to tumorigenesis, and the management of these patients can be based on a series of serum markers such as SII, NLR, white blood cell count (WBC) white blood cell count, and C-reactive protein level (CRP) that can evaluate and demonstrate the efficiency of the pharmacological and surgical treatment. Due to the complex interactions between various immune-inflammatory conditions and cancer, higher prognostic predictive performance can be achieved if a comprehensive IIB that includes more responses to the overall immune-inflammatory state is used.
Recently, a more complex immune-inflammation index has been proposed, encompassing four parameters: neutrophils, lymphocytes, monocytes, and platelets, and referred to as the pan-immune-inflammation value (PIV) [11]. PIV has been shown to have prognostic value in a variety of tumors, such as esophageal squamous cell carcinoma [12], breast cancer [13] and prostate cancer [14]. However, fewer studies have explored its association with postoperative prognosis in OSCC, leaving its prognostic role in this context unclear. Therefore, the aim of this study was to evaluate the prognostic performance of preoperative PIV in patients with OSCC after radical resection and to develop prognostic columnar plots for individual survival prediction.
Materials and methods
Study design and study population
In this retrospective cohort study, 1,146 patients diagnosed with primary oral cancer at the First Affiliated Hospital of Fujian Medical University (Fuzhou City, Fujian Province, China) from January 2015 to March 2022 were recruited for follow-up and blood tests. Patients who met the following inclusion criteria were recruited: (a) patients aged between 20 and 80 years old and treated with radical resection; (b) histologically confirmed primary OSCC; and (c) residence in Fujian Province for at least 10 years. We excluded participants with (a) recurrent OSCC, systemic inflammatory diseases (e.g., hepatitis, nephritis, and prolonged immunosuppression), or hematologic disorders (e.g., anemia, leukemia, and lymphoma); (b) prior treatment that could affect preoperative PIV (e.g., chemotherapy, radiotherapy, and immunosuppressive drugs); and (c) perioperative death. A total of 1146 patients were included, and 779 OSCC patients were ultimately recruited, of which 367 were excluded due to not meeting the inclusion criteria or having insufficient information (Supplementary Fig. 1).
Data collection and definition
Patient demographics, including age, weight, height, gender, place of residence, education, smoking, and alcohol consumption, were collected through a structured questionnaire for interviews. Clinical characteristics of the patients, including tumor site, stage, tumor differentiation, complications, postoperative hospital stay, and duration of surgery, were obtained through the electronic medical record system. Tumor staging was determined according to the TNM classification of malignant tumors (8th edition) [15, 16], and tumor differentiation was defined according to the World Health Organization guidelines [17].
In addition, laboratory data on neutrophil counts, platelet counts, monocyte counts, and lymphocyte counts were collected from all patients within one week prior to the procedure. The PIV values were calculated from neutrophil counts, platelet counts, monocyte counts, and lymphocyte counts using the formula neutrophil counts*platelet counts*monocyte counts/lymphocyte counts. The optimal PIV cutoff value was calculated using Yale University X-tile software.
Follow-up
Telephone, outpatient follow-up or electronic medical record tracking was performed every 6 months after discharge. Information about patients of loss of follow-up was collected by checking medical records of readmission. Patients who failed to follow up three times in a row at various intervals within 1 week or were still alive were attributed to censors. The study ended on September 22, 2022, with a minimum follow-up threshold of 6 months. Overall survival (OS) was from cancer diagnosis to death or study end, whichever came first.
Ethical considerations
Written informed consent was obtained from all enrolled participants. This research was performed in accordance with the ethical standards of the Helsinki Declaration, and the ethical approval was obtained from the Ethics Committees of Fujian Medical University, Fuzhou, China (approval ID: 2011053).
Statistical analysis
Continuous variables are presented as median (IQR) and categorical variables are presented as numerical and frequency distributions. The statistical significance of baseline characteristics before and after matching was determined using standardized mean deviation (SMD) or t-tests. The proportional risk hypothesis was tested using Schoenfeld residuals. To identify independent factors affecting OS, we performed univariate and multivariate Cox regression analyses, calculated hazard ratios (HR) and 95% confidence intervals (CI), and performed Kaplan-Meier survival curves and log-rank tests. Based on continuous PIV values, restricted cubic spline (RCS) modeling was used to investigate the non-dose-response relationship between PIV and OS.
To better assess the relationship between pretreatment PIV and OSCC prognosis, we used propensity score matching (PSM) to balance potential confounders across different PIV level groups. Briefly, using PIV level as the outcome of a multivariate logistic regression model with all relevant confounding variables (including age, gender, BMI, place of residence, education, smoking, alcohol consumption, cancer site, TNM stage, tumor differentiation, hypertension, diabetes mellitus, postoperative radiotherapy, postoperative chemotherapy, Post-operative hospitalization, surgery duration, and comorbidities). This model allowed us to calculate a propensity score for each OSCC patient. In the PSM process, a nearest neighbor matching algorithm with a caliper value of 0.2 was applied to match individuals with differing PIV levels on a 1:1 basis, resulting in 249 cases in each group. Subsequently, survival analysis was conducted to further assess the prognostic significance of PIV.
Finally, nomogram modeling was employed to predict the prognostic survival of OSCC patients and compared with conventional TNM staging, in conjunction with Randomized Survival Forest (RSF) to determine the predictive importance of PIV (compared with other parameters) for the survival of OSCC patients. All statistical analyses were conducted using R software (version 4.4.0), with a two-sided P value of less than 0.05 deemed statistically significant.
Result
Patient characteristics
Patient characteristics are shown in Table 1. A total of 779 patients who underwent oral cancer surgery were recruited in this study. A total of 2,185 person-years of follow-up up to July 2022, 632 cases were censored (comparising 519 surviving cases and 113 lost to follow-up, resulting in a loss rate of 14.51%), and 147 deaths were recorded, with a mean follow-up time of 34.14 ± 24.39 months. The mean age of all patients was 61.00 years (IQR: 52.00–69.00), and 305 (39.15%) were females and 474 (60.85%) were males. According to the clinical staging, most of the patients were in Stage IV (40.08%). The median PIV was 174.26 (IQR: 97.93- 297.95), and according to the X-tile software, a PIV cutoff value of 180.90 was obtained and patients were categorized into low PIV (≤ 180.9) or high PIV (> 180.9) group.
Table 1.
Patient baseline demographic
| All (%/median [IQR]) | Survival situation | ||
|---|---|---|---|
| Censored (%) | Dead (%) | ||
| n | 779 | 632 | 147 |
| Age (Year) | 61.00 [52.00,69.00] | 61.00 [52.00,68.00] | 62.00 [54.00,70.00] |
| Gender | |||
| Female | 305(39.15) | 247(39.08) | 58(39.46) |
| Male | 474(60.85) | 385(60.92) | 89(60.54) |
| Tobacco use | |||
| No | 457(58.66) | 378(59.81) | 79(53.74) |
| Yes | 322(41.34) | 254(40.19) | 68(46.26) |
| Alcohol consumption | |||
| No | 523(67.14) | 426(67.41) | 97(65.99) |
| Yes | 256(32.86) | 206(32.59) | 50(34.01) |
| Hypertension | |||
| No | 558(71.63) | 457(72.31) | 101(68.71) |
| Yes | 221(28.37) | 175(27.69) | 46(31.29) |
| Diabetes | |||
| No | 680(87.29) | 558(88.29) | 122(82.99) |
| Yes | 99(12.71) | 74(11.71) | 25(17.01) |
| Cancer site | |||
| Oral tongue | 365(46.85) | 301(47.63) | 64(43.54) |
| Gums | 87(11.17) | 68(10.76) | 19(12.93) |
| Flour of mouth | 108(13.86) | 91(14.40) | 17(11.56) |
| Others | 219(28.11) | 172(27.22) | 47(31.97) |
| TNM stage | |||
| I | 148(19.00) | 138(21.84) | 10(6.80) |
| II | 161(20.67) | 145(22.94) | 16(10.88) |
| III | 150(19.26) | 128(20.25) | 22(14.97) |
| IV | 320(41.08) | 221(34.97) | 99(67.35) |
| Tumor differentiation | |||
| Well | 281(36.07) | 246(38.92) | 35(23.81) |
| Moderate | 363(46.60) | 288(45.57) | 75(51.02) |
| Poor | 135(17.33) | 98(15.51) | 37(25.17) |
| BMI(Kg/m2) | |||
| >=18.5;<=24 | 493(63.29) | 404(63.92) | 89(60.54) |
| < 18.5 | 78(10.01) | 50(7.91) | 28(19.05) |
| > 24 | 208(26.70) | 178(28.16) | 30(20.41) |
| Postoperative radiotherapy | |||
| No | 543(69.70) | 443(70.09) | 100(68.03) |
| Yes | 236(30.30) | 189(29.91) | 47(31.97) |
| Postoperative chemotherapy | |||
| No | 415(53.27) | 333(52.69) | 82(55.78) |
| Yes | 364(46.73) | 299(47.31) | 65(44.22) |
| Complications | |||
| No | 538(69.06) | 466(73.73) | 72(48.98) |
| Yes | 241(30.94) | 166(26.27) | 75(51.02) |
| Duration of surgery (hours) | 5.17[3.42,7.52] | 4.95[3.30,7.17] | 6.83[4.55,8.67] |
| Post-operative hospitalization(days) | 11.62[8.59,14.56] | 11.60[8.56,14.49] | 12.61[9.26,14.85] |
| PIV | 174.26[97.93,297.95] | 162.14[94.72,276.73] | 219.91[127.56,388.95] |
Prognostic analysis of PIV
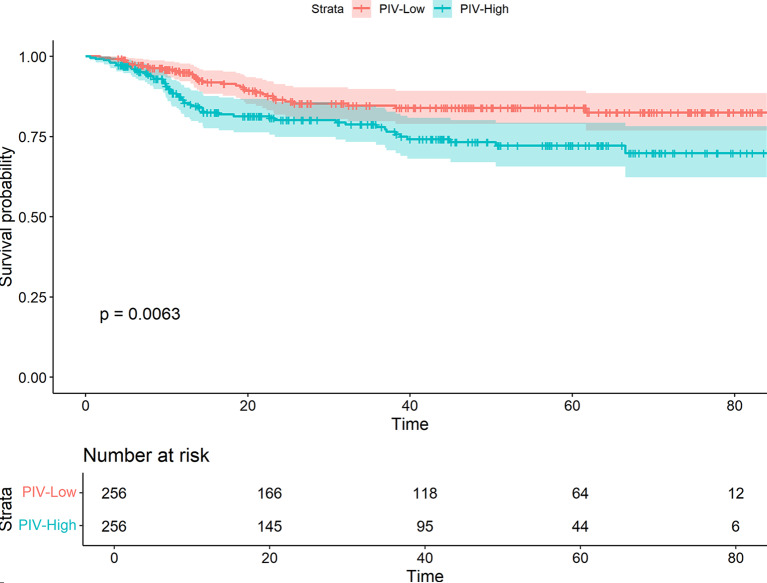
In order to evaluate the prognostic influencing factors of oral cancer, this study conducted unifactorial and multifactorial analyses of oral cancer OS. In the unifactorial results (Supplemental Table 1), age, diabetes mellitus, clinical stage, degree of differentiation, BMI, PIV, comorbidities, and duration of surgery were the potential influencing factors related to oral cancer OS, and the above variables were included in the multifactorial Cox regression analyses. The results showed that age was positively associated with the prognosis of oral cancer (HR = 1.02, 95%CI:1.01–1.04, P = 0.009), the risk of death in patients with clinical stage IV was 3.13 times higher than that in patients with stage I (HR = 3.13, 95%CI:1.56–6.28, P = 0.001), and the risk of death in patients with poorly differentiated oral cancer was 1.63 times higher than that in patients with highly differentiated oral cancer (HR = 1.63, 95%CI:1.02–2.60, P = 0.043), and patients with BMI < 18.5Kg/m2 had an 81% increased risk of death compared with normal weight patients (HR = 1.81, 95%CI:1.17–2.28, P = 0.008) Postoperative complications were also associated with increased mortality risk (HR = 1.84, 95% CI:1.30–2.60, P = 0.001). Furthermore, higher PIV values were positively correlated with increased risk of death, with the high PIV group exhibiting a 1.62-fold higher risk compared to the low PIV group (HR = 1.62, 95%CI:1.15–2.29, P = 0.006) (Table 2). Figure 1 shows a significant survival difference (P < 0.001) between the low PIV group (PIV ≤ 180.9) and the high PIV group (PIV > 180.9).
Table 2.
Multivariate Cox regression analysis of OS
| Variables | HR (95%CI) | P |
|---|---|---|
| Age | 1.02(1.01–1.04) | 0.009 |
| Diabetes | ||
| No | 1.00 | |
| Yes | 1.47(0.94–2.30) | 0.088 |
| TNM stage | ||
| I | 1.00 | |
| II | 1.25(0.56–2.78) | 0.585 |
| III | 1.64(0.76–3.55) | 0.207 |
| IV | 3.13(1.56–6.28) | 0.001 |
| Tumor differentiation | ||
| Well | 1.00 | |
| Moderate | 1.46(0.97–2.20) | 0.069 |
| Poor | 1.63(1.02–2.60) | 0.043 |
| BMI(Kg/m2) | ||
| >=18.5;<=24 | 1.00 | |
| < 18.5 | 1.81 (1.17, 2.80) | 0.008 |
| > 24 | 0.82 (0.54, 1.24) | 0.345 |
| PIV | ||
| Low | 1.00 | |
| High | 1.62(1.15–2.29) | 0.006 |
| Complications | ||
| No | 1.00 | |
| Yes | 1.84(1.30–2.60) | 0.001 |
| Duration of surgery | 1.03(0.96–1.10) | 0.390 |
Fig. 1.
The Kaplan–Meier curve of low and high level of PIV
Additionally, to explore the dose-response relationship between PIV values and the prognostic risk of oral cancer, a restricted cubic spline plot based on the raw PIV values was generated (Supplemental Fig. 2), which showed a linear association between PIV and prognostic risk (P For Nonlinear = 0.8561) .
PSM-based validation
To address the differences in baseline characteristics, we performed PSM based on the PIV grouping. Supplementary Table 2 presents the baseline characteristics before and after matching. Prior to matching, patients with a high PIV value were more likely to be male, smoke, not drink alcohol, have an oral cancer site of the tongue, be classified as stage IV, experience longer surgical durations, and have extended hospital stays following surgery. After matching, these variables were balanced and comparable, as demonstrated by the standardized mean differences (SMD) depicted in Supplementary Fig. 3. Following matching, the Kaplan-Meier curve (Figs. 2) indicated a continued survival difference between the high PIV value group and the low PIV value group (P = 0.0.34), and the multifactorial Cox regression after matching showed that (Table 3), age, stage, BMI, postoperative complications, and PIV value remained significant risk factors for oral cancer prognosis. Specifically, the risk of death was 1.55 times higher in the high PIV group compared to the low PIV group (HR = 1.55 times higher in the low PIV group). 1.55 times (HR = 1.55, 95%CI:1.03–2.23, P = 0.035), a finding consistent with results observed before matching.
Fig. 2.
The Kaplan–Meier curve of low and high level of PIV after matching
Table 3.
Multivariate Cox regression after PSM
| Variables | HR(95%CI) | P |
|---|---|---|
| Age | 1.02(1.00–1.04) | 0.020 |
| Diabetes | ||
| No | 1.00 | |
| Yes | 0.92(0.48–1.76) | 0.808 |
| TNM stage | ||
| I | 1.00 | |
| II | 1.25(0.47–3.34) | 0.651 |
| III | 1.86(0.74–4.68) | 0.185 |
| IV | 3.67(1.59–8.49) | 0.002 |
| Tumor differentiation | ||
| Well | 1.00 | |
| Moderate | 1.12(0.69–1.82) | 0.649 |
| Poor | 1.16(0.65–2.09) | 0.618 |
| BMI(Kg/m2) | ||
| >=18.5&<=24 | 1.00 | |
| < 18.5 | 2.10(1.17–3.76) | 0.013 |
| > 24 | 0.91(0.55–1.49) | 0.702 |
| PIV | ||
| Low | 1.00 | |
| High | 1.55(1.03–2.33) | 0.035 |
| Complications | ||
| No | 1.00 | |
| Yes | 1.78(1.16–2.74) | 0.009 |
| Duration of surgery | 1.00(0.92–1.10) | 0.920 |
Randomized survival forest analysis
The significance value of each predictor variable in the RSF analysis was evaluated using the log-rank split rule, with a higher significance value indicating greater predictive power. As illustrated in Fig. 3, TNM stage, PIV value, duration of surgery, complications, BMI, postoperative hospitalization, postoperative radiotherapy, diabetes mellitus, degree of differentiation, hypertension, postoperative chemotherapy, tumor site, age, gender, and smoking were identified as significant predictors (variable importance > 0). Among these, PIV values were ranked third in predictive importance, indicating that PIV values play a crucial role in predicting the prognosis of oral cancer.
Fig. 3.
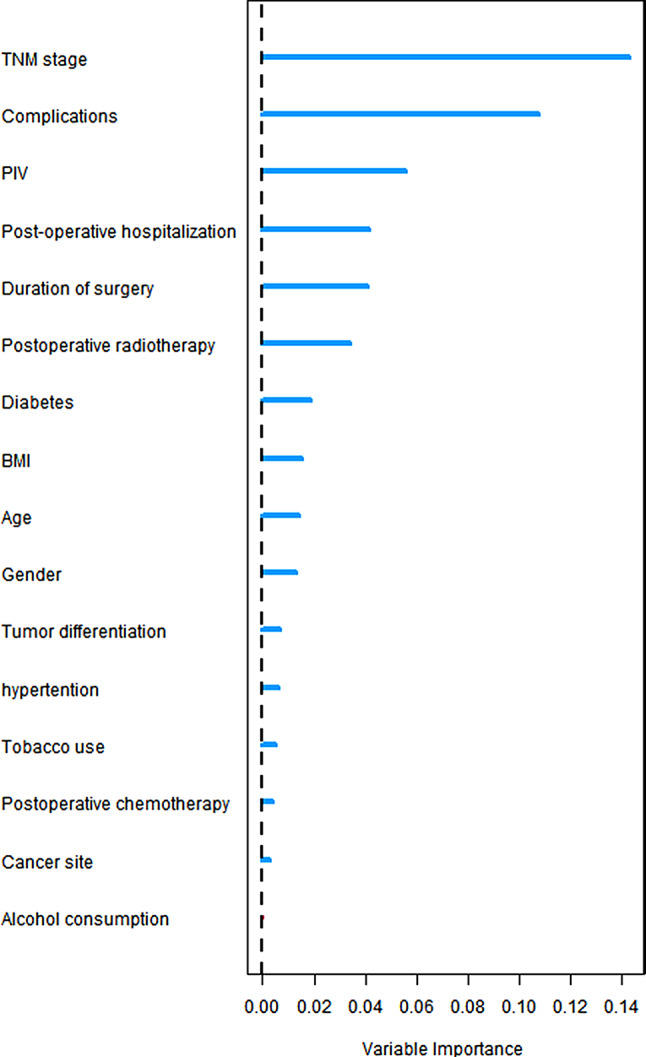
Importance of stochastic survival forest variables
Building nomogram models
We constructed a prognostic Nomogram model for oral cancer patients based on age, clinical stage, degree of differentiation, diabetes mellitus, comorbidities, duration of surgery, PIV value, and BMI (Fig. 4). Further plotted ROC curves to compare the prognostic risk prediction ability of death of oral cancer with the traditional TNM staging (Fig. 5), which showed that the Nomogram model constructed by combining PIV value, exhibited strong predictive performance for 1-, 3-, and 5-year survival rates of oral cancer patients, with AUC values of 0.796, 0.754, and 0.756, respectively, and outperformed the predictive ability of traditional TNM. The internally validated calibration curves (Fig. 6) showed the good performance of the Nomogram model in predicting 1-, 3-, and 5-year survival rates of oral cancer.
Fig. 4.
Nomogram model based on GLR and other significant predictors
Fig. 5.
Comparison of prediction performance between PIV-based model and index TNM in OS
Fig. 6.
Calibration curves for predicting prognostic 1-(A), 3-(B), and 5-year (C) survival rates PIV-based models
Discussion
In this study, we reviewed a prospective cohort of 779 OSCC patients with the aim of investigating the prognostic value of PIV in oral cancer patients after surgery, and among the results, age, TNM stage, tumor differentiation, BMI, PIV, and comorbidities were observed to be significant prognostic predictors. More importantly, higher PIV values were associated with an elevated risk of death from oral cancer, and similar findings were observed in the matched cohort, where propensity score-based cohorts minimized differences in the distribution of confounding variables between groups. In addition, results of Randomized Survival Forest analysis showed that PIV was only less important than TNM staging, suggesting that it is a valuable prognostic predictor. The column-line graph model, incorporating PIV and other factors, demonstrated superior predictive performance compared to the traditional TNM model, with calibration curves confirming its effectiveness in predicting 1-, 3-, and 5-year survival rates for oral cancer.
Cancer-associated inflammation is prevalent in malignant tumors and is closely related to tumorigenesis, progression, and prognosis. Thus, it has long been considered a biomarker of cancer as well [18]. PLR, NLR, MLR, and SII, which are based on two or three inflammatory markers, do not fully capture the immunoinflammatory state. PIV is composed of four inflammatory markers and is calculated as neutrophil count (109/L) × monocyte count (109/L) × platelet count (109/L)/lymphocyte count (109/L). PIV may provide a more comprehensive picture of the immunoinflammatory status, and its components can be obtained by routine preoperative blood tests, thus PIV may be a reliable and cost-effective biomarker in clinical practice. Our study found that oral cancer patients with high PIV values had poorer prognoses compared to those with low PIV values, consistent with findings from other studies [13, 19, 20]. These results were also validated by the PSM cohort, which also showed similar results. Considering that there will be some heterogeneity across studies that may have different cutoff values, a meta-analysis synthesizing 30 studies showed that [21], Cancer patients in the high PIV group had significantly worse OS (HR = 2.07; 95% CI:1.77–2.41; I2 = 73.0%). Of course, we also performed restricted cubic spline analysis using consecutive PIV and observed a linear dose-response relationship between PIV and prognostic mortality in oral cancer patients (Supplementary Fig. 2).
While the precise mechanisms by which PIV influences malignancy prognosis are not fully understood, it is suggested that higher PIV values reflect increased levels of neutrophils, monocytes, and platelets, and decreased levels of lymphocytes. Neutrophils are innate immune cells that are most abundant in the bone marrow and peripheral blood [22]. Not only can they release reactive oxygen species (ROS), reactive nitrogen or proteases to promote carcinogenesis, but they can also induce angiogenesis and secrete chemokines (e.g. VEGFA and MMP9) to tend to tumor invasion and metastasis [23]. Monocytes are progenitors of macrophages and a subclass of dendritic cells [24]. Can be recruited into the tumor microenvironment (TME) where they undergo local differentiation and will be activated as tumor-associated macrophages (TAM) [25], and promotes tumor cell invasion and angiogenesis by secreting various cytokines (e.g., TNF-α, IL-1β, IL-8) [26]. Platelets interact with tumor cells by releasing growth factors and small molecules that facilitate tumor growth and invasion, while tumor cells can activate platelets, leading to aggregation and granule release [27]. Finally, lymphocytes, as a type of immune cell, play an important role in the anti-tumor immune process by inducing apoptosis and inhibiting the value-added and migration of tumor cells [28]. Although the prognostic value of PIV in oral cancer remains to be fully elucidated, studies have shown that [29], higher SII values, which include three of the four components of PIV, are associated with reduced overall survival in OSCC patients who underwent radical resection, and SII values are composed of three of the four cell types that make up PIV; therefore, higher levels of SII values imply higher levels of inflammation, which could plausibly point to an indirect link between high PIV and poorer prognosis of patients with oral cancer, which would be in line with the results of the present study, which, in turn, more fully interprets the inflammatory state of the host. The Randomized Survival Forest results of this study conclude that PIV is a valuable predictor after TNM staging for oral cancer prognosis, and the fact that the blood parameter is a simple and routine indicator also implies that PIV is convenient and cost-effective; moreover, the column-line graphical model developed based on clinical and demographic factors such as PIV can quickly and graphically predict OSCC patients’ Prognosis.
This study has some limitations. Firstly, due to sample size limitations, we did not consider the response of inflammation in different HP subtypes of oral cancer. Secondly, the study was conducted at a single center, and larger multicenter studies are needed. The Nomogram model was only validated within this population, and future research should include diverse patient cohorts from different regions and ethnicities to test its generalizability. Lastly, PIV values may change over time, and dynamic assessment could more accurately represent a patient’s immune status compared to a single-time point measurement, which would enhance PIV’s prognostic value.
Conclusion
PIV is an independent prognostic factor with oral squamous cell carcinoma, and the Nomogram model incorporating PIV effectively predicts prognosis. Further large-scale prospective studies are necessary to validate our findings and explore the underlying mechanisms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
B.C. He were involved in the conceptualization and design of the study. W.H. Huang and Y.L. Lin drafted the work or substantively revised it. W.H. Huang, E.L. Xu and Yanmei Ji were involved in conducting statistical analyses. J. Wang, F.Q. Liu, and F. Chen were responsible for creating figures and tables. Y. Qiu, B. Shi and L.S. Lin were involved in data collection and interpretation. All authors were involved in the critical revision of the manuscript.
Funding
This study was supported by Fujian Natural Science Foundation Program (Grant/award nos. 2022J01235, and 2022J01239), and Mutual Expert Research Project of the First Affiliated Hospital of Fujian Medical University (Grant/award no. YJRCHP- 2023HBC).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from all enrolled participants. This research was performed in accordance with the ethical standards of the Helsinki Declaration, and the ethical approval was obtained from the Ethics Committees of Fujian Medical University, Fuzhou, China (approval ID: 2011053).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang F, Niu X, Zhou M, et al. Development and validation of a novel disulfidptosis-related lncRNAs signature in patients with HPV-negative oral squamous cell carcinoma [J]. Sci Rep. 2024;14(1):14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldoni R, Scolaro A, Boccalari E et al. Malignancies and biosensors: a focus on oral Cancer detection through salivary biomarkers [J]. Biosens (Basel). 2021;11(10). [DOI] [PMC free article] [PubMed]
- 3.Roi A, Roi CI, Andreescu NI, et al. Oral cancer histopathological subtypes in association with risk factors: a 5-year retrospective study [J]. Rom J Morphol Embryol. 2020;61(4):1213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Ranz M, Lequerica-Fernández P, Rodríguez-Santamarta T, et al. Prognostic implications of preoperative systemic inflammatory markers in oral squamous cell carcinoma, and correlations with the local immune tumor microenvironment [J]. Front Immunol. 2022;13:941351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma–an update [J]. CA Cancer J Clin. 2015;65(5):401–21. [DOI] [PubMed] [Google Scholar]
- 6.Tsai YT, Fang KH, Tsai MH, et al. Prognostic utility of preoperative platelet-to-albumin ratio in surgically treated oral cavity cancer patients [J]. Head Neck. 2024;46(2):386–97. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa T, Iga T, Takeda D, et al. Neutrophil-lymphocyte ratio associated with poor prognosis in oral cancer: a retrospective study [J]. BMC Cancer. 2020;20(1):568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients [J]. Br J Cancer. 2014;110(10):2524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H, Liu Q, Zhu L, et al. Prognostic value of preoperative systemic Immune-inflammation index in patients with cervical Cancer [J]. Sci Rep. 2019;9(1):3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roi CI, Roi A, Nicoară A, et al. Impact of treatment on systemic Immune-Inflammatory Index and other inflammatory markers in Odontogenic Cervicofacial Phlegmon cases: a retrospective study [J]. Biomedicines. 2023;11(6):1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fucà G, Guarini V, Antoniotti C, et al. The Pan-immune-inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials [J]. Br J Cancer. 2020;123(3):403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng J, Wang L, Yang X, et al. Clinical utility of preoperative pan-immune-inflammation value (PIV) for prognostication in patients with esophageal squamous cell carcinoma [J]. Int Immunopharmacol. 2023;123:110805. [DOI] [PubMed] [Google Scholar]
- 13.Lin F, Zhang LP, Xie SY, et al. Pan-immune-inflammation Value: a New Prognostic Index in operative breast Cancer [J]. Front Oncol. 2022;12:830138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yazgan SC, Yekedüz E, Utkan G, et al. Prognostic role of pan-immune-inflammation value in patients with metastatic castration-resistant prostate cancer treated with androgen receptor-signaling inhibitors [J]. Prostate. 2022;82(15):1456–61. [DOI] [PubMed] [Google Scholar]
- 15.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer staging Manual: continuing to build a bridge from a population-based to a more personalized approach to cancer staging [J]. CA Cancer J Clin. 2017;67(2):93–9. [DOI] [PubMed] [Google Scholar]
- 16.Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual [J]. CA Cancer J Clin. 2017;67(2):122–37. [DOI] [PubMed] [Google Scholar]
- 17.Almangush A, Mäkitie AA, Triantafyllou A, et al. Staging and grading of oral squamous cell carcinoma: an update [J]. Oral Oncol. 2020;107:104799. [DOI] [PubMed] [Google Scholar]
- 18.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer [J]. Cell. 2010;140(6):883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Şahin AB, Cubukcu E, Ocak B, et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy [J]. Sci Rep. 2021;11(1):14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guven DC, Erul E, Yilmaz F, et al. The association between pan-immune-inflammation value and survival in head and neck squamous cell carcinoma [J]. Eur Arch Otorhinolaryngol. 2023;280(5):2471–8. [DOI] [PubMed] [Google Scholar]
- 21.Hai-Jing Y, Shan R, Jie-Qiong X. Prognostic significance of the pretreatment pan-immune-inflammation value in cancer patients: an updated meta-analysis of 30 studies [J]. Front Nutr. 2023;10:1259929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis [J]. J Hematol Oncol. 2021;14(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ocana A, Nieto-Jiménez C, Pandiella A, et al. Neutrophils in cancer: prognostic role and therapeutic strategies [J]. Mol Cancer. 2017;16(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson A, Han CZ, Glass CK, et al. Monocyte regulation in Homeostasis and malignancy [J]. Trends Immunol. 2021;42(2):104–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ugel S, Canè S, De Sanctis F, et al. Monocytes in the Tumor microenvironment [J]. Annu Rev Pathol. 2021;16:93–122. [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application [J]. J Hematol Oncol. 2017;10(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haemmerle M, Stone RL, Menter DG, et al. The platelet lifeline to Cancer: challenges and opportunities [J]. Cancer Cell. 2018;33(6):965–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Huang W, Wu Y, et al. Prognostic role of pretreatment blood lymphocyte count in patients with solid tumors: a systematic review and meta-analysis [J]. Cancer Cell Int. 2020;20:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diao P, Wu Y, Li J, et al. Preoperative systemic immune-inflammation index predicts prognosis of patients with oral squamous cell carcinoma after curative resection [J]. J Transl Med. 2018;16(1):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.