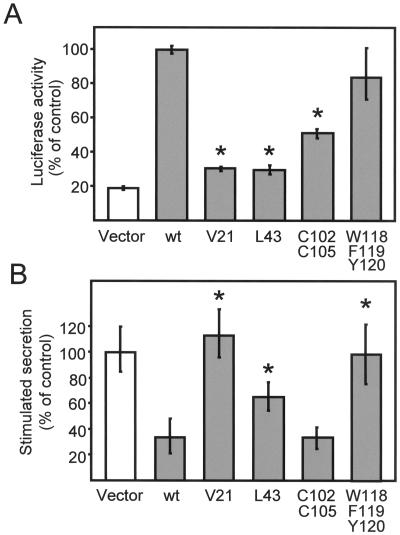
Figure 7.
Effect of different point mutations of granuphilin on Rab3 binding and on exocytosis. (A) HIT-T15 cells were cotransfected with a fusion protein of GAL4 with the GTPase-deficient mutant Rab3AQ81L, a fusion protein of VP16 with the indicated mutants of granuphilin-b and a plasmid containing five binding sites for GAL4 upstream of the firefly luciferase gene. The interaction of the GTP-bound form of Rab3A with the mutants of granuphilin-b was assessed by measuring firefly luciferase activity. The luciferase activity produced in cells expressing the fusion proteins of GAL4 with Rab3AQ81L and VP16 with wild-type (wt) granuphilin-b was set to 100%. The results are the mean ± SD of three independent experiments performed in duplicate. Asterisks indicate the mutants whose interaction with Rab3A is significantly (p < 0.01) different from wild-type granuphilin-b. (B) HIT-T15 cells were transiently transfected with the indicated mutants of granuphilin-b and with a plasmid encoding hGH. After 3 days in culture, the cells were incubated under basal conditions or in the presence of depolarizing concentrations of K+ and glucose. The amount of hGH released into the medium under basal and stimulatory conditions was determined by ELISA. The response of the cells transfected with the hGH plasmid together with an empty vector (vector) was set to 100%. The figure shows the mean ± SD of at least five independent experiments measured in triplicate. Asterisks indicate the mutants whose effect on exocytosis is significantly (p < 0.01) different from that of wild-type granuphilin-b.