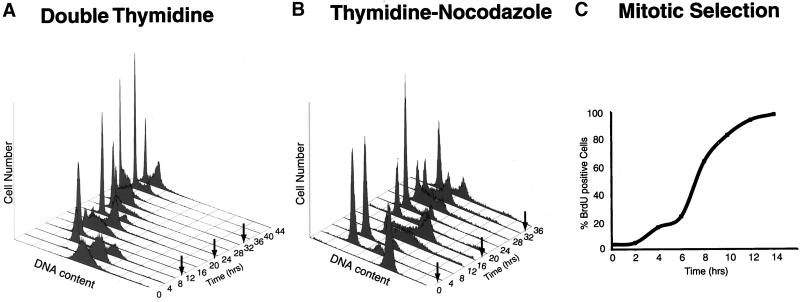
Figure 1.
Synchrony of HeLa cells. (A) Cells were arrested at the beginning of S phase by using a double thymidine block, and cell synchrony was monitored by flow cytometry of propidium iodide-stained cells. Flow cytometry data were collected for each of the three independent double thymidine blocks performed in this study; data are shown only for the second double thymidine arrest (Thy-Thy2), although equivalent synchrony was obtained in each of the three experiments. The number of cells (arbitrary units) is plotted against DNA content for time points at 4-h intervals for 44 h; an arrow indicates the time of mitosis, as estimated from the flow cytometry data. Upon release from the thymidine block, >95% of the cells progressed into S phase (0–4 h), entered G2 phase (5–6 h), underwent a synchronous mitosis at 7–8 h, and reentered S phase after completing one full cell cycle at 14–16 h. Typically two to three additional synchronous cell cycles were obtained. (B) Cells were arrested in mitosis by blocking first in thymidine followed by release and then blocking in nocodazole. After release from the nocodazole block, most of the cells (>75%) divided synchronously within 2 h of release from the arrest, entered S phase by 10–12 h after release, and completed the next synchronous mitosis by 18–20 h, ultimately completing two full cell cycles. (C) Cells were selected by mitotic shake-off, and the percentage of cells in S phase was measured by BrdU incorporation. The percentage of cells in S phase is plotted against the time after plating; >95% of the cells entered S phase by 12 h.