Abstract
Background
Due to its excellent acidifier, antioxidant, and preservative properties, citric acid is added to or is a constituent in a broad range of foods and beverages. Its measurement should be performed to assure the food quality specifications are met.
Objective
To validate the performance of the Enzytec™ Liquid Citric acid test kit for the determination in food and beverages such as wines, juices, and tomato products.
Methods
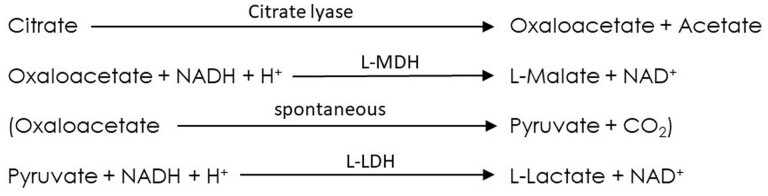
The kit contains two ready-to-use components, which makes handling very easy and suitable for automation. Citrate is cleaved into oxaloacetate and acetate by citrate lyase. Oxaloacetate reacts to L-malate by L-malate dehydrogenase and reduced nicotinamide adenine dinucleotide (NADH). Pyruvate, spontaneously formed from oxaloacetate, is converted by L-lactate dehydrogenase to L-lactate. The NADH consumed is equivalent to the amount of citric acid converted and is measured at a wavelength of 340 nm within 20 min.
Results
The test is specific to citric acid and shows no relevant interferences. Limit of detection and limit of quantification are 15 and 40 mg/L, respectively when using a test volume of 100 µL. The linear measurement range is from 40 to 1000 mg/L citric acid. Trueness was evaluated using materials from FAPAS, NIST, LGC, and two control wines from the German Wine Analysts. Matrix interference was evaluated by spiking tomato ketchup, tomato paste, orange juice, and carbonated beverages and resulted in recoveries around 100%. Intermediate precision is between 6.2 and 8.5% for matrixes with extraction and below 4% for matrixes measured directly. For automation, three applications with different test volumes and different measurement ranges were validated. Linearity is given from 8 mg/L up to 5 g/L (depending on the test volume).
Conclusions
The method is robust and accurate for manual and automated applications. The method was approved as an AOAC Official Method of Analysis℠.
Highlights
The ready-to-use components of the test kit have a shelf life of at least 24 months from the date of manufacture.
Due to its excellent acidifier, antioxidant, and preservative properties, citric acid (citrate; food additives E330–E333 according to EU regulation EC/1333/2008) is found in a broad range of foods and beverages, such as fruit juice and other (carbonated) soft drinks, beer, bread, candies, and dairy or meat products. Furthermore, it is used in the wine industry, with an allowable upper limit of just 1 g/L according to the Organisation Internationale de la vigne et du vin (OIV) code of practice (1). The quantification of citric acid is also important in clinical chemistry for measurement in urine or seminal plasma (2, 3).
AOAC Official Method℠ 985.11 (citric acid in wine) is being considered for removal from the Official Methods of Analysis, because the test kit Citric Acid (Cat. No. 10 139 076 035) previously supplied by Boehringer/Roche is no longer available. Roche decided to discontinue the so-called Yellow Line test kits. The new Enzytec™ Liquid Citric acid was created as a replacement for the Roche test kit. In parallel it was decided to refurbish enzymatic analysis per se. Ready-to-use reagents with a long shelf life and the possibility for automated use were the most important requirements from the market.
Experimental
The manufacturer’s in-house validation scheme followed a former validation study for ethanol in kombucha and other beverages, which led to Official Method 2017.07 First and Final Action (4, 5) and long-lasting practical experiences of the method developer for ready-to-use enzymatic test kits.
Linearity.—Linearity checked over a range from 20 up to 1600 mg/L citric acid (in water) using three different test kit lots with two replicates in each lot. This experiment was also performed for the automated analyzer in a similar way.
Limits of detection (LOD) and limit of quantification (LOQ) (estimates).—According to DIN 32645:2008–11 (based on DIN ISO 11843–2:2008–06) with concentrations ranging from 10 up to 100 mg/L citric acid (in water) analyzed using three independent test kit lots (n = 2 replicates per lot).
LOQ.—Derived out of data from a precision profile. This experiment was also performed for the automated analyzer in a similar way.
Precision profile.—Data were calculated from the linearity data set (aqueous citric acid solutions). The calculated RSD values are derived from two replicates from three independent test kit lots each.
Selectivity.—Experiment using L-ascorbic acid, D-tartaric acid, D/L-malic acid, D/L-isocitric acid, L-tartaric acid, L- and D-lactic acid, acetic acid, meso-tartaric acid, and oxalic acid at a concentration equivalent to 5.2 mmole/L of citric acid (1.0 g/L). All solutions were prepared in distilled water.
Interferences.—Different sugars, sugar substitutes, artificial sweeteners, and organic acids were tested in the presence of 500 mg/L citric acid. Meso-tartaric acid and sulfite were tested in a concentration range between 1.56 and 25 g/L in the presence of 500 mg/L citric acid. D/L-malic acid was tested between 3.13 up to 50 g/L.
Trueness.—Measurement of two certified reference materials (LGC juice organic acids and a NIST cranberry juice) with 12 replicates on 1 day using one test kit lot and tested by one analyst.
Recovery.—Three different matrixes (tomato ketchup, orange juice, tomato paste) were spiked to their endogenous citric acid concentrations or lower or higher; test portions were extracted and diluted in 12 replicates and tested by one analyst. For three different carbonated soft drinks, samples were individually spiked below and above their natural citric acid content. Test portions were degassed, centrifuged, and tested in n = 6 on one day in one test kit lot. This experiment was also performed for the automated analyzer in a similar way.
Repeatability.—A tomato paste and a tomato ketchup were tested over a period of three days by one person, with one extract per day of which six replicates were tested; repeatability was also characterized during characterization for intermediate precision. This experiment was also performed for the automated analyzer in a similar way.
Inter-lot precision.—Aqueous citric acid solutions containing 1000 and 250 mg/L and a reference wine containing 1005 mg/L of citric acid were analyzed with six replicates on one day by one person using three different test kit lots.
Intermediate precision.—Measurement of a FAPAS control soft drink, a NIST cranberry juice, two reference wines, ketchup, and orange juice; each material was used directly for measurement or extracted before and analyzed using one test kit lot by three different analysts on two different days with two replicates per measurement. From these values sr, RSDr, si, and RSDi were calculated. This design also allowed for an evaluation of the contribution of analyst, day, and extraction and pipetting steps to the overall precision.
Robustness.—Incubation temperature was varied between 18 and 37°C. Incubation time before measuring A2 at 340 nm was varied between 5 and 20 min. An orange juice, a tomato ketchup a tomato paste, and a spiked wine with concentrations above the measurement range were checked for dilutability within the measurement range.
Stability.—All three test kit lots were stored at 2 to 8°C for up to 24 months and tested at regular intervals. One test kit lot was used as an assessment of open kit stability in parallel.
AOAC Official Method 2024.02
Citric Acid in Selected Foods and Beverages
Enzytec™ Liquid Citric acid: Manual and
Automated Applications
First Action 2024
[Applicable for the quantitative measurement of citric acid in fruit juices, (carbonated) soft drinks, wines, tomato ketchup, and tomato concentrate (paste). SO2 and meso-tartaric acid do not interfere at or below 3.13 g/L. A concentration of 50 mg/L of pyruvic acid will react and result in a decrease of the upper linear limit by 0.11 g/L citric acid. Under practical conditions, there will be no significant influence of results for citric acid if naturally occurring amounts of pyruvic acid are present.]
Caution: See Material Safety Data Sheet and Instructions for Use available at https://eifu.r-biopharm.com/food/US/food.
A. Principle
The enzymatic reaction requires three enzymes (citrate lyase, malate dehydrogenase, and lactate dehydrogenase) and NADH. Citrate present in the test solution is cleaved into oxaloacetate and acetate by citrate lyase (CL). In the presence of L-malate dehydrogenase (L-MDH) and reduced nicotinamide adenine dinucleotide (NADH), oxaloacetate reacts to L-malate, whereby NADH is consumed. If oxaloacetate de-carboxylates, the formed pyruvate will react with NADH to L-lactate by L-lactate dehydrogenase (L-LDH) (Figure 2024.02A).
Figure 2024.02A.
Enzymatic reaction scheme for determination of citric acid.
The NADH consumed is equivalent to the converted amount of citric acid consumed and is measured at a wavelength of 340 nm within 20 min. The result is expressed as g/L, g/kg, mg/L, or g/L of citric acid. The test kit only contains two ready-to-use components, which forms the basis for the robust, precise, and simple quantification of citric acid.
B. Chemicals and Reagents
Items (a)–(b) are available as a test kit (Enzytec Liquid Citric acid, E8230; R-Biopharm AG, Darmstadt, Germany). Refer to kit label for expiry at 2 to 8°C (36 to 46°F) and date of production.
Reagent 1.—2 × 50 mL containing L-MDH + L-LDH and NADH in buffer.
Reagent 2.—2 × 12.5 mL containing CL in buffer.
Enzytec Multi acid standard high.—5000 mg/L of citric acid, acetic acid, L-malic acid, D-malic acid, L-lactic acid, D-lactic acid, and D-gluconic acid each; E8465, R-Biopharm, Darmstadt, Germany.
-
Enzytec Multi acid standard low.—250 mg/L of citric acid, acetic acid, L-malic acid, D-malic acid, L-lactic acid, D-lactic acid, and D-gluconic acid each; E8460, R-Biopharm, Darmstadt, Germany.
Required but not provided with the test kit:
Distilled water.
Potassium hydroxide, 1 M.—Solubilized in water; do not store in glass vials and keep plastic containers closed to prevent reaction with carbon dioxide.
Polyvinylpolypyrrolidone (PVPP).—e.g., Anafin Soft P, ZEFÜG GmbH & Co. KG, Bingen, Germany.
C. Apparatus
Apparatus specified has been tested. Equivalent apparatus may be used.
Analytical balance.—Entris 623i-1S, Sartorius Lab Instruments, Goettingen, Germany.
pH meter.—FiveEasy™ pH/mV bench meter, Mettler-Toledo, Giessen, Germany.
Beakers.
Fluted paper filters.
Magnetic stirrer.—Cimarec™ Poly 15, Fisher Scientific, Schwerte, Germany.
Graduated flasks.—50, 100, 200 mL.
Reaction tubes.—2 mL; re-closable.
Syringe filters.—Minisart® High Flow syringe filter 16532 K, 0.22 µm Polyethersulfon, Sartorius, Goettingen, Germany.
Disposable plastic cuvettes.—1 cm light path.
Micropipettors.—20–200 and 100–1000 μL.
Multipette.—To dispense 2 mL aliquots of reagent 1 and 500 µL of reagent 2 for manual pipetting.
Spectrophotometer capable of reading at 340 nm.—Cary 60, UV-Vis Spectrophotometer, Agilent Technologies, Waldbronn, Germany or equivalent. For manual application.
Analyzer.—Pictus 500 (Diatron, Budapest, Hungary) or equivalent. For automated application.
Vortex mixer.
Ultrasound device.—Sonorex Super RK 31, Bandelin, Berlin, Germany.
Centrifuge.—Mikrozentrifuge 5427 R, Eppendorf SE, Hamburg, Germany.
D. General Preparations
Store the kit at 2–8°C (36–46°F). Let all kit components come to room temperature 20–25°C (68–77°F) before use. Do not freeze any of the kit components.
Use separate tips for each test solution (and control solutions) to avoid cross-contamination, and pre-flush the tip before pipetting.
Use a multi-stepper pipette for adding the reagent 1 and reagent 2 solution. Use a single tip for each of these components.
Components and procedures of the test kit have been standardized for use in this procedure. Do not interchange components between kits of different batches (lot numbers).
Store laboratory samples in a cold and dry room protected from light. Ensure that no cross-contamination takes place.
Keep in mind that solid matrixes can be inhomogeneous; therefore, grind a representative part of the laboratory sample very well and homogenize before weighing.
E. Sample Preparation
Use clear test samples directly, or after dilution with distilled water to a citric acid concentration between 40 and 1000 mg/L. For test solutions with concentrations close to the limit of quantification (LOQ), it is recommended to increase the test volume (see G(g)).
For turbid test samples.—Filter by using fluted paper filter/syringe filter or centrifuge the test samples in a reaction tube (recommended 3000 g for at least 5 min) until a clear filtrate or supernatant is obtained.
Degas test samples containing carbon dioxide by aid of an ultrasound burst.
Adjust pH value of strongly acidic test samples (wine and juices) by adding 1 M KOH to a known sample volume until pH value is between 6.5 and 7.5; bring to a known volume and dilute further if necessary with distilled water.
Tomato ketchup.—Accurately weigh approximately 1 g of test sample (±1 mg) in a 100 mL beaker, add about 25 mL of distilled water, and stir for about 10 min on a magnetic stirrer with a magnetic stir bar. Transfer this suspension quantitatively into a 50 mL volumetric flask and dilute to 50 mL with water. Mix the content of the flask and filter through a paper filter (discard the first 15 mL) or use a syringe filter; alternatively, centrifuge at 3000 g for at least 5 min in a reaction tube until a clear supernatant is obtained.
Tomato concentrate.—Accurately weigh approximately 1 g of test sample (±1 mg) in a 100 mL beaker, add about 25 mL of water, and stir for about 10 min on a magnetic stirrer with a magnetic stir bar. Transfer this suspension quantitatively into a 100 mL volumetric flask and dilute to 100 mL with distilled water. Mix the content of the flask and filter through a paper filter (discard the first 15 mL) or use a syringe filter; alternatively centrifuge at 3000 g for at least 5 min in a reaction tube until a clear supernatant is obtained.
Use PVPP when testing juices/wines with a strong dark color that are measured undiluted: Add 0.1 g PVPP to 10 mL of juice or wine, stir/shake for 1 min, and filter (fluted paper filter or syringe filter) or centrifuge at 3000 g for at least 5 min in a reaction tube until a clear supernatant is obtained.
F. Determination
For a test volume of 100 µL in the manual application, the linear range is between 40 and 1000 mg/L. The limit of detection (LOD) is 15 mg/L. For automated applications, the measurement ranges are from 40 to 1200 mg/L (basic), 500 to 5000 mg/L (high range), and 8 to 100 mg/L (sensitive).
-
Manual using 4 mL cuvettes
It is recommended to use controls such as references or standard solutions (e.g., referenced in B(d)). No calibrators are needed for the manual application.
Pipet the test or control solutions (such as reagent d) with a variable micropipet and the reagent 1 and 2 solution with a multi-stepper pipet to ensure good mixing.
Insert a sufficient number of cuvettes in a holder for all test solution or controls, for single determination. Record test solutions and control positions.
With each measurement, it is necessary to determine a reagent blank (RB) by using distilled water instead of test solution or control solution.
Pipet 2 mL of reagent 1 (R1) in each cuvette.
Add 100 µL of distilled water (blank), test solution, or control solution; mix carefully (e.g., using disposable plastic spatulas).
Incubate for 3 min between 20 and 37°C.
Read and document absorbance A1 in a spectrophotometer set at 340 nm for each cuvette.
Add 500 µL of reagent 2 (R2) in each cuvette and mix well.
Incubate for 15 min between 20 and 37°C (64–77°F).
Read and document absorbance A2 in a spectrophotometer set at 340 nm for each cuvette.
In case of higher (control or) test volumes (up to 1000 µL), the volumes for R1 and R2 remain unchanged; the calculation must be adjusted as described in G; check pH value of the test sample and neutralize the pH in case of any doubt; test solutions must be clear.
-
Automation on a Pictus 500 device
Calibration is required when using the Pictus device. To give a user the maximum flexibility, three different applications with different measurement concentration ranges depending on the test volume of 2, 10, and 100 µL are provided. Volumes of reagent 1 and reagent 2 were reduced to 200 and 50 µL, respectively, and are not changed for the three different applications.
High range application.—Two-point linear calibration with 0 mg/L (distilled water) and 5000 mg/L (use Enzytec Multi acid standard high, described in B(c)).
Basic range application.—Two-point linear calibration with 0 mg/L (distilled water) and 1000 mg/L (use Enzytec Multi acid standard high, described in B(c)) after dilution 1 + 4 with distilled water).
Sensitive range application.—Four-point calibration with 0, 11.1, 33.3, and 100 mg/L prepared by diluting Enzytec Multi Acid standard (B(d)) with distilled water.
To ensure that these calibrations are valid over a longer period (e.g., one week), the respective aqueous control solutions (e.g., B(c) and (d)) have to be analyzed with every run. In case these control solutions are not within specifications given in H(b), a re-calibration must be done.
-
Determination
(a) Add 200 µL reagent 1.
-
(b) Add test solution, control, or calibrator/water; volumes depend on the application:
2 µL for high range application
10 µL for basic range application
100 µL for sensitive application
(c) It is recommended to pipet n = 4 replicates for each calibrator.
(d) Incubate for 2 min at 37 °C.
(e) Read A1 at 340 nm.
(f) Add 50 µL reagent 2.
(g) Incubate for 10 min at 37 °C.
(h) Read A2 at 340 nm.
Recommendations for other automated analyzers.—The ratio of 4:1 for reagent 1 and 2 should not be changed, and the test volume should not be bigger than twice the volume of reagent 2. A calibration must be performed but is often stable for several days, so it does not need to be repeated on every day. Control solution(s) should be analyzed with every run to check the validity of the calibration. If these control solutions are not within specifications, a recalibration must be done.
G. Calculations
-
Calculate ΔA for every test sample or control:where df is a dilution factor depending on the test volume:
-
Calculate concentrations for every test sample or control (100 µL test volume):where V = final volume; MW = molecular weight of citric acid; ε = absorption coefficient of NADH at 340 nm; d = light path within cuvette; v = test volumeor
If the test solution was diluted before measurement, this result has to be multiplied with the dilution factor.
-
Using a test volume of 1000 µL, the calculation will change as follows:or
- Calculation in solid test samples:
Calculations for automated analysis.—The Pictus 500 device will calculate a calibration function from the calibrators and use this function to calculate concentrations for unknown test solutions and control solutions.
-
Results close to LOQ.—If the result is between 100 and 150 mg/L for a test volume of 100 µL, repeat measurement with 200 µL, and if lower than 100 mg/L, repeat measurement with 500 µL or 1000 µL test volume for better accuracy (trueness, precision).
Measurement range for 200 µL test volume: 20 to 500 mg/L.
Measurement range for 1000 µL test volume: 4 to 100 mg/L.
H. System Suitability Tests/Analytical Quality Control
For each analytical run, an aqueous control solution (e.g., Enzytec Multi acid standard low, 250 mg/L citric acid) should be analyzed.
Recoveries for an aqueous control solution should be within the 95 to 105% range.
-
Use matrix-containing control samples or Certified Reference Materials, e.g.,
NIST Standard Reference Material 3282 Low Calorie Cranberry Juice Cocktail, 3221 mg/kg citric acid ± 53 mg/kg, k = 2
FAPAS Quality Control Material Soft Drink (T03167QC); assigned value 2870 mg/L; 150 mL
Standardwein der dtsch. Weinanalytiker (https://www.weinanalytiker.de/standard-testloesung/); standard wine of the German Wine Analysts, e.g., label “orange” lot 1081608: 1.005 +/- 0.0373 g/L of citric acid; k = 1 or label “moosgrün” lot 1071505: 0.457 +/- 0.0123 g/L; k = 1.
If results do not fall within this range, (1) check data sets for any suspicious values (e.g., decreased A1 values or unexpected high variation), (2) check incubation temperature and time, (3) check pipets for accuracy, and (4) check photometer for correct wavelength.
Results and Discussion
General Remarks
The following sections will contain the characterization of the manual format using 4 mL cuvettes. These sections will contain all necessary performance characteristics as required by AOAC and are based on a former validation study for ethanol in kombucha and other beverages, which led to Official Method 2017.07 Final Action (4, 5). In addition, the Diatron Pictus 500 spectrophotometric auto-analyzer was taken as one example of an automated pipetting device that is used regularly in laboratories performing enzymatic analysis. Since some performance characteristics are independent of the way the test kit is used, each single performance characteristic was not repeated for both manual and automated applications.
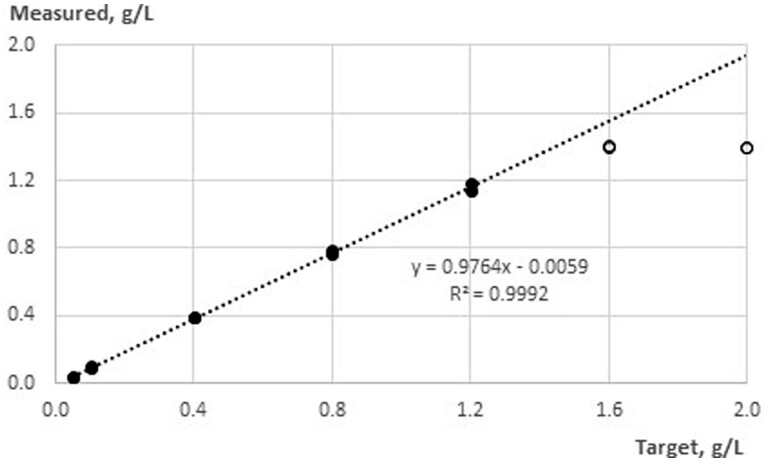
Linearity/Measurement Range
Figure 1 shows the characterization of linearity using mean data from three lots with two independent runs each for concentrations from 20 up to 1600 mg/L. For calculation of the linear regression, only data for concentrations between 20 and 1400 mg/L were used. The resulting formula is
Figure 1.
Linearity of the system between 20 and 1600 mg/L out of data derived from three lots with n = 2 replicates and a test volume of 100 µL; the open circle was not included in linear regression.
From the plot shown in Figure 1, it is clear that the upper limit of linearity is 1400 mg/L for a new test kit lot, and the lower limit of linearity is the LOQ (40 mg/L; see Estimation of Limit of Quantification) by definition. It should be noted that stored test kit lots will not reach 1400 mg/L, e.g., after 18 months of storage at 2 to 8 °C, an upper linear limit between 1000 and 1200 mg/L was observed (data not shown); therefore, a practical upper range of linearity of 1000 mg/L is stated in the IFU to cover the whole shelf life. An experiment to characterize the whole measurement range when using a higher test volume was not performed for citric acid in the manual application but for the automated version (high range application).
Estimation of LOD
The LOD was determined according to DIN 32645 (comparable to DIN ISO 11843–2). A detailed description of the calculation can be found in AOAC Official Method 2017.05 (4). Each measurement was tested with 10 different aqueous citric acid solutions, with concentrations between 10 and 100 mg/L citric acid and a test volume of 100 µL. This set of dilutions was tested two times independently with each lot (Table 1). An LOD of 15 mg/L of citric acid for a test volume of 100 µL was calculated.
Table 1.
Results from estimation of limit of detection in all three lots for 100 µL test volume
| Lot | Estimated according to DIN 32645, mg/L |
|---|---|
| 1 | 12.7 |
| 2 | 13.2 |
| 3 | 13.1 |
The LOD experiment was repeated in lot TC3 with an increased test volume of 1000 µL and a concentration range between 1 and 10 mg/L (see Figure 2). The LOD calculated according to DIN 32645 is 1.61 mg/L. A similar experiment will also be performed on the Diatron Pictus 500 analyzer (see Automation on a Pictus 500 Spectrophotometric Analyzer).
Figure 2.
Measured A340 nm (ΔOD) values for aqueous solution with concentrations between 1 and 10 mg/L for two independent measurements for the pilot scale lot TC3; test volume was 1000 µL.
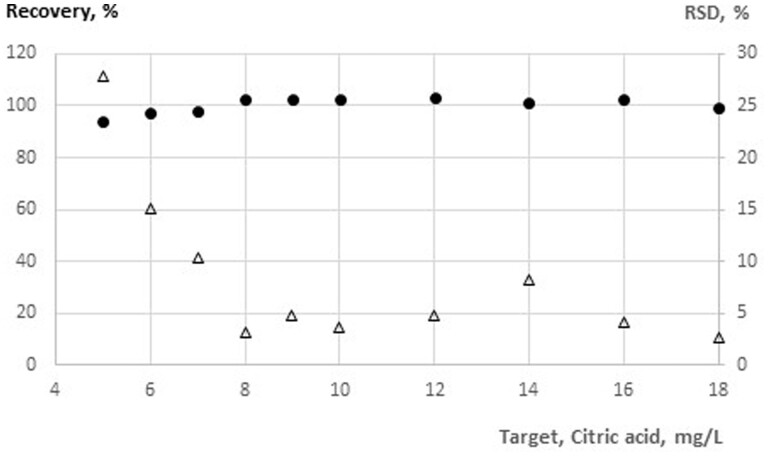
Estimation of LOQ
The LOQ is also determined according to DIN 32645 (comparable to DIN ISO 11843–2). Each measurement was tested with 10 different aqueous citric acid solutions with concentrations between 10 and 100 mg/L citric acid. This set of dilutions was tested two times independently with each lot (Table 2). Results in Table 2 led to the assumption that the LOQ is between 20 and 25 mg/L, but data in Figure 3 clearly show that at a citric acid level of 20 mg/L the RSD is still at 15% and that the citric acid concentration with a RSD at 10% is 40 mg/L. It was therefore decided to state an LOQ of 40 mg/L citric acid for a test volume of 100 µL.
Table 2.
Results from estimation of limit of quantification in all three test kit lots in comparison to the prototype for 100 µL test volume
| Lot | Estimated according to DIN 32645, mg/L |
|---|---|
| 1 | 21.3 |
| 2 | 22.2 |
| 3 | 21.9 |
| Prototype | 20.0 |
Figure 3.
Measured mean ΔA340 nm (ΔOD) values for aqueous solution with concentrations between 10 and 100 mg/L for two independent measurements for each lot (closed circles) and a test volume of 100 µL; the relative standard deviation in % is also given (open triangles).
Taking the data from the LOD experiment with a test volume of 1000 µL, the calculated LOQ is 2.66 mg/L. It should be noted that using the 1000 µL approach will result in higher standard deviations (Figure 2).
Selectivity: Side Activity
To show the high selectivity of the citrate lyase reaction, the following substances/substrates were tested for side reactivity: L-ascorbic acid, D-tartaric acid, D/L-malic acid, D/L-isocitric acid, L-tartaric acid, L- and D-lactic acid, acetic acid, meso-tartaric acid, and oxalic acid. None of them showed side-activity (Table 3). The substances were further tested at higher concentrations for characterization of interference (Tables 4 and 5).
Table 3.
Side activity toward important organic acids at a concentration of 5.2 mmole/L, which is equivalent to 1 g/L of citric acid
| Side activity | A340 nm |
|||||
|---|---|---|---|---|---|---|
| Substance | Concna, g/L | Vol., mL | A 1 | A2 (15 min) | A340 nm | Citric acid, g/L |
| Water | –b | 0.1 | 2.122 | 1.709 | 0.004 | – |
| Ascorbic acid | 0.916 | 0.1 | 2.132 | 1.709 | 0.009 | 0.007 |
| D-Tartaric acid | 0.780 | 0.1 | 2.134 | 1.715 | –0.003 | –0.002 |
| DL-Malic acid | 1.395 | 0.1 | 2.128 | 1.711 | 0.003 | 0.003 |
| Na3-DL-Isocitrate*H20 | 2.684 | 0.1 | 2.141 | 1.720 | –0.002 | –0.002 |
| L-Tartaric acid | 0.780 | 0.1 | 2.125 | 1.714 | –0.002 | –0.001 |
| Li-L-Lactate | 0.499 | 0.1 | 2.132 | 1.710 | 0.000 | 0.000 |
| Na-D-Lactate | 0.583 | 0.1 | 2.129 | 1.711 | 0.004 | 0.003 |
| Na-Acetate | 0.427 | 0.1 | 2.128 | 1.713 | –0.006 | –0.005 |
| meso-Tartaric acid*H20 | 0.874 | 0.1 | 2.126 | 1.712 | 0.001 | 0.001 |
| Oxalic acid*2 H20 | 0.656 | 0.1 | 2.135 | 1.711 | 0.002 | 0.001 |
5.2 mmole/L for each substance.
– = Not applicable.
Table 4.
Substances tested for interference in the presence of citric acid
| Interference | Citric acid |
|||||||
|---|---|---|---|---|---|---|---|---|
| Substance | Concn, g/L | Vol., mL | Target, g/L | A 1 | A2 (15 min) | ΔA340 nm | Measured, g/L | Rec., % |
| D-Fructose | 25 | 0.1 | 0.5 | 2.135 | 1.077 | 0.621 | 0.492 | 98.5 |
| D-Glucose | 25 | 0.1 | 0.5 | 2.128 | 1.075 | 0.636 | 0.504 | 100.9 |
| Sucrose | 25 | 0.1 | 0.5 | 2.129 | 1.073 | 0.627 | 0.497 | 99.5 |
| Lactose | 25 | 0.1 | 0.5 | 2.131 | 1.074 | 0.628 | 0.498 | 99.5 |
| Cyclamate | 25 | 0.1 | 0.5 | 2.127 | 1.075 | 0.617 | 0.489 | 97.8 |
| Sucralose | 25 | 0.1 | 0.5 | 2.125 | 1.087 | 0.621 | 0.492 | 98.4 |
| Xylitol | 25 | 0.1 | 0.5 | 2.135 | 1.074 | 0.624 | 0.495 | 98.9 |
| Saccharin | 3.22a | 0.1 | 0.5 | 2.134 | 1.074 | 0.630 | 0.500 | 100.0 |
| Acesulfam K | 25 | 0.1 | 0.5 | 2.134 | 1.071 | 0.644 | 0.510 | 102.1 |
| D-Sorbitol | 25 | 0.1 | 0.5 | 2.124 | 1.073 | 0.623 | 0.494 | 98.8 |
| L-Ascorbic acid | 25 | 0.1 | 0.5 | 2.136 | 1.074 | 0.625 | 0.495 | 99.0 |
| D-Tartaric acid | 25 | 0.1 | 0.5 | 2.133 | 1.078 | 0.636 | 0.505 | 100.9 |
| L-Tartaric acid | 25 | 0.1 | 0.5 | 2.132 | 1.072 | 0.643 | 0.509 | 101.9 |
| D-Lactic acid | 25 | 0.1 | 0.5 | 2.075 | 1.024 | 0.643 | 0.510 | 102.0 |
| L-Lactic acid | 25 | 0.1 | 0.5 | 2.130 | 1.079 | 0.615 | 0.488 | 97.6 |
| Sorbic acid | 0.8a | 0.1 | 0.5 | 2.129 | 1.060 | 0.640 | 0.507 | 101.5 |
| Acetic acid | 25 | 0.1 | 0.5 | 2.131 | 1.075 | 0.626 | 0.496 | 99.2 |
| NaCl | 25 | 0.1 | 0.5 | 2.121 | 1.073 | 0.614 | 0.487 | 97.4 |
Limited solubility in water.
Table 5.
Substances tested in more detail for interference in the presence of citric acid
| Interference | Citric acid |
|||||||
|---|---|---|---|---|---|---|---|---|
| Substance | Concn, g/L | Vol., mL | Target, g/L | A 1 | A2 (15 min) | ΔA340 nm | Measured, g/L | Rec., % |
| meso-Tartaric acid | 25 | 0.1 | 0.5 | 2.133 | 1.350 | 0.365 | 0.290 | 57.9 |
| meso-Tartaric acid | 12.5 | 0.1 | 0.5 | 2.134 | 1.212 | 0.503 | 0.398 | 79.7 |
| meso-Tartaric acid | 6.25 | 0.1 | 0.5 | 2.132 | 1.125 | 0.588 | 0.466 | 93.2 |
| meso-Tartaric acid | 3.13 | 0.1 | 0.5 | 2.129 | 1.070 | 0.641 | 0.508 | 101.7 |
| meso-Tartaric acid | 1.56 | 0.1 | 0.5 | 2.135 | 1.075 | 0.640 | 0.508 | 101.5 |
| D-/L-Malic acid (sum of both) | 50 | 0.1 | 0.5 | 2.051 | 1.150 | 0.487 | 0.386 | 77.3 |
| D-/L-Malic acid (sum of both) | 25 | 0.1 | 0.5 | 2.130 | 1.071 | 0.616 | 0.489 | 97.7 |
| D-/L-Malic acid (sum of both) | 12.5 | 0.1 | 0.5 | 2.133 | 1.075 | 0.616 | 0.488 | 97.6 |
| D-/L-Malic acid (sum of both) | 6.25 | 0.1 | 0.5 | 2.135 | 1.074 | 0.618 | 0.490 | 98.0 |
| D-/L-Malic acid (sum of both) | 3.13 | 0.1 | 0.5 | 2.131 | 1.069 | 0.619 | 0.491 | 98.2 |
| SO2 (as Na2SO3) | 25 | 0.1 | 0.5 | 2.131 | 1.153 | 0.560 | 0.444 | 88.7 |
| SO2 (as Na2SO3) | 12.5 | 0.1 | 0.5 | 2.133 | 1.095 | 0.596 | 0.472 | 94.4 |
| SO2 (as Na2SO3) | 6.25 | 0.1 | 0.5 | 2.130 | 1.094 | 0.594 | 0.471 | 94.1 |
| SO2 (as Na2SO3) | 3.13 | 0.1 | 0.5 | 2.135 | 1.091 | 0.600 | 0.476 | 95.1 |
| SO2 (as Na2SO3) | 1.56 | 0.1 | 0.5 | 2.136 | 1.082 | 0.610 | 0.484 | 96.8 |
Pyruvic acid as a part of the reaction cascade (see A) to determine citric acid was not investigated in practice but as a theoretical calculation. First, pyruvic acid present in the measurement solution will react when adding reagent 1. Therefore, it will only limit the upper measurement range. A concentration of 50 mg/L of pyruvic acid (equivalent to 0.5678 mmole/L) will react and result in the conversion of 0.5678 mmole/L of NADH. Using the formula given in G(b) and solving it for ΔA, this results in a theoretical decrease of A1 (around 2.1) as shown in Table 1 by 0.138. This will decrease the upper linear limit by 0.138 × 0.7929 equals to 0.11 g/L citric acid [see formula given in G(b)]. Naturally occurring pyruvic acid concentrations are between 0.097 mg/L for tomatoes and 11.6 mg/L for lager beer (6). Under practical conditions, there will be no significant influence of results for citric acid if naturally occurring amounts of pyruvic acid are present.
Selectivity Study: Interference
Table 4 shows the substances that have been tested for assay interference. A concentration of, for example, 25 g/L of the interfering substance was mixed with 0.5 g/L citric acid solution and the recovery was then determined. In the case of sulfur dioxide and meso-tartaric acid (Table 5), there is no interference observed at or below 3.13 g/L. The sum of D- and L-malic acid does not interfere at or below 25 g/L (Table 5). The other substances do not have an interfering effect in the determination of citric acid.
Trueness
The trueness of the enzymatic test system was also checked during the characterization for intermediate precision. Furthermore, it was checked with two Certified Reference Materials (LGC Dr Ehrenstorfer Fruit Juice Organic Acid Mixture (CRM); DRE-GS09000056WA; 2023 mg/L citric acid with a measurement uncertainty of 18 mg/L and NIST Standard Reference Material 3282 Low Calorie Cranberry Juice Cocktail; 3221 mg/kg citric acid ± 0.053 mg/kg, k = 2).
With an RSD value around 1%, the mean recovery is at 100% or close to 105% (Table 6). Both materials had to be diluted before measurement. Using the procedure described in the ERM Application Note no. 1 (available at https://crm.jrc.ec.europa.eu/graphics/cms_docs/erm1_english.pdf), both data sets were checked if there were significant differences to the certified value—this was not the case (7).
Table 6.
Repeated measurement (n = test portions) of two Certified Reference Materials for characterization of trueness in one lot
| LGC juice organic acid target, 2.032 g/L |
NIST cranberry juice target, 3.221 g/L |
|||
|---|---|---|---|---|
| Replicate | g/L | Rec., % | g/L | Rec., % |
| 1 | 2.047 | 100.7 | 3.450 | 107.1 |
| 2 | 2.017 | 99.3 | 3.338 | 103.6 |
| 3 | 2.042 | 100.5 | 3.393 | 105.3 |
| 4 | 2.063 | 101.5 | 3.378 | 104.9 |
| 5 | 2.037 | 100.3 | 3.384 | 105.1 |
| 6 | 2.044 | 100.6 | 3.334 | 103.5 |
| 7 | 2.109 | 103.8 | 3.381 | 105.0 |
| 8 | 2.042 | 100.5 | 3.380 | 105.0 |
| 9 | 2.044 | 100.6 | 3.397 | 105.5 |
| 19 | 2.032 | 100.0 | 3.340 | 103.7 |
| 11 | 2.061 | 101.4 | 3.384 | 105.1 |
| 12 | 2.052 | 101.0 | 3.390 | 105.2 |
| Mean, g/L | 2.049 | 100.8 | 3.379 | 104.9 |
| SD, g/L | 0.0226 | 0.0316 | ||
| RSD, % | 1.10 | 0.94 | ||
Recovery Using Spiked Matrixes
This performance characteristic was tested during validation for tomato ketchup, orange juice, tomato paste, and three different carbonated beverages because no (certified) reference materials were available for these matrixes. Tomato products and orange juice showed quite high endogenous citric acid contents, as can be seen in the upper part of Tables 7–9. Each matrix was extracted/diluted as described in E with six biological replicates each. Each of the existing extracts was measured twice so that, in total, 12 results were obtained. These extracts were also used for spiking purposes and measured. For spiking, 90% volume of the extract was mixed with 10% volume of the spiking solution, which explains the “90% value” in the top of Tables 7–9. It was decided to spike levels that were at or half of the original citric acid contents.
Table 7.
Results of naturally incurred and spiked matrix samples; naturally incurred tomato ketchup sample extracts were spiked to result in a sample concentration of about 12 g/kg
| Tomato ketchup | ||||
|---|---|---|---|---|
| Dilution | Spike, g/kg | Measured, g/kg | 90%, value g/kg | |
| 50 | 0 | 6.38 | 5.74 | |
| 50 | 0 | 6.75 | 6.07 | |
| 50 | 0 | 6.75 | 6.07 | |
| 50 | 0 | 6.58 | 5.92 | |
| 50 | 0 | 6.88 | 6.19 | |
| 50 | 0 | 6.92 | 6.23 | |
| 50 | 0 | 6.53 | 5.87 | |
| 50 | 0 | 6.23 | 5.61 | |
| 50 | 0 | 6.54 | 5.88 | |
| 50 | 0 | 6.39 | 5.75 | |
| 50 | 0 | 6.51 | 5.86 | |
| 50 | 0 | 6.63 | 5.97 | |
| Mean | 6.59 | |||
| SD | 0.21 | |||
| RSD, % | 3.14 | |||
|
| ||||
| Spike, g/kga | Rec., % | |||
|
| ||||
| 50 | 6.48 | 12.11 | 6.37 | 98.3 |
| 50 | 6.48 | 12.09 | 6.02 | 92.9 |
| 50 | 6.48 | 12.27 | 6.20 | 95.7 |
| 50 | 6.48 | 11.67 | 5.75 | 88.8 |
| 50 | 6.48 | 12.32 | 6.13 | 94.6 |
| 50 | 6.48 | 12.15 | 5.92 | 91.4 |
| 50 | 6.48 | 12.60 | 6.73 | 103.8 |
| 50 | 6.48 | 12.51 | 6.90 | 106.5 |
| 50 | 6.48 | 12.37 | 6.48 | 100.1 |
| 50 | 6.48 | 12.33 | 6.57 | 101.4 |
| 50 | 6.48 | 12.44 | 6.59 | 101.7 |
| 50 | 6.48 | 12.73 | 6.77 | 104.4 |
| Mean | 6.37 | 98.3 | ||
| SD | 0.36 | 5.63 | ||
| RSD, % | 5.73 | |||
Measured (spiked) – 90% value.
Table 8.
Results of naturally incurred and spiked matrix samples; naturally incurred orange juice sample extracts were spiked to result in a sample concentration of about 11 and 15 g/L
| Orange juice | ||||
|---|---|---|---|---|
| Dilution | Spike, g/L | Measured, g/L | 90% value, g/L | |
| 25 | 0 | 7.87 | 7.08 | |
| 25 | 0 | 8.02 | 7.22 | |
| 25 | 0 | 8.08 | 7.27 | |
| 25 | 0 | 7.92 | 7.13 | |
| 25 | 0 | 8.61 | 7.75 | |
| 25 | 0 | 7.85 | 7.07 | |
| 25 | 0 | 8.30 | 7.47 | |
| 25 | 0 | 8.41 | 7.57 | |
| 25 | 0 | 8.37 | 7.53 | |
| 25 | 0 | 8.09 | 7.28 | |
| 25 | 0 | 9.02 | 8.12 | |
| 25 | 0 | 8.39 | 7.55 | |
| Mean | 8.25 | |||
| SD | 0.34 | |||
| RSD, % | 4.18 | |||
|
| ||||
| Spike, g/kga | Rec., % | |||
|
| ||||
| 25 | 4 | 10.87 | 3.79 | 94.6 |
| 25 | 4 | 11.09 | 3.87 | 96.8 |
| 25 | 4 | 10.95 | 3.67 | 91.9 |
| 25 | 4 | 11.13 | 4.01 | 100.1 |
| 25 | 4 | 11.37 | 3.63 | 90.6 |
| 25 | 4 | 10.98 | 3.91 | 97.8 |
| 25 | 4 | 11.41 | 3.94 | 98.5 |
| 25 | 4 | 11.37 | 3.80 | 95.0 |
| 25 | 4 | 11.49 | 3.95 | 98.8 |
| 25 | 4 | 11.30 | 4.02 | 100.4 |
| 25 | 4 | 11.99 | 3.87 | 96.7 |
| 25 | 4 | 11.50 | 3.95 | 98.8 |
| Mean | 3.87 | 96.7 | ||
| SD | 0.12 | 3.10 | ||
| RSD, % | 3.21 | |||
|
| ||||
| 25 | 8 | 14.58 | 7.49 | 93.7 |
| 25 | 8 | 14.78 | 7.56 | 94.4 |
| 25 | 8 | 15.18 | 7.91 | 98.9 |
| 25 | 8 | 14.74 | 7.61 | 95.1 |
| 25 | 8 | 16.31 | 8.56 | 107.0 |
| 25 | 8 | 14.86 | 7.79 | 97.4 |
| 25 | 8 | 14.93 | 7.46 | 93.2 |
| 25 | 8 | 15.21 | 7.64 | 95.5 |
| 25 | 8 | 15.28 | 7.74 | 96.8 |
| 25 | 8 | 15.22 | 7.94 | 99.3 |
| 25 | 8 | 16.17 | 8.05 | 100.6 |
| 25 | 8 | 15.16 | 7.61 | 95.1 |
| Mean | 7.78 | 97.2 | ||
| SD | 0.31 | 3.84 | ||
| RSD, % | 3.95 | |||
Measured (spiked) – 90% value.
Table 9.
Results of naturally incurred and spiked matrix; naturally incurred tomato paste sample extracts were spiked to result in a sample concentration of about 40 and 60 g/kg
| Tomato paste | ||||
|---|---|---|---|---|
| Dilution | Spike, g/kg | Measured, g/kg | 90% value, g/kg | |
| 100 | 0 | 20.70 | 18.63 | |
| 100 | 0 | 22.55 | 20.30 | |
| 100 | 0 | 21.29 | 19.16 | |
| 100 | 0 | 21.17 | 19.05 | |
| 100 | 0 | 21.73 | 19.56 | |
| 100 | 0 | 21.91 | 19.72 | |
| 100 | 0 | 21.18 | 19.06 | |
| 100 | 0 | 21.00 | 18.90 | |
| 100 | 0 | 19.96 | 17.96 | |
| 100 | 0 | 21.14 | 19.02 | |
| 100 | 0 | 21.64 | 19.47 | |
| 100 | 0 | 21.39 | 19.25 | |
| Mean | 21.30 | |||
| SD | 0.64 | |||
| RSD, % | 3.02 | |||
|
| ||||
| Spike, g/kga | Rec., % | |||
|
| ||||
| 100 | 21 | 39.66 | 21.03 | 100.1 |
| 100 | 21 | 38.30 | 18.00 | 85.7 |
| 100 | 21 | 38.95 | 19.79 | 94.2 |
| 100 | 21 | 39.36 | 20.30 | 96.7 |
| 100 | 21 | 39.95 | 20.39 | 97.1 |
| 100 | 21 | 39.05 | 19.33 | 92.1 |
| 100 | 21 | 40.22 | 21.15 | 100.7 |
| 100 | 21 | 40.89 | 21.99 | 104.7 |
| 100 | 21 | 40.42 | 22.46 | 106.9 |
| 100 | 21 | 40.43 | 21.40 | 101.9 |
| 100 | 21 | 39.37 | 19.89 | 94.7 |
| 100 | 21 | 40.63 | 21.37 | 101.8 |
| Mean | 20.59 | 98.1 | ||
| SD | 1.23 | 5.88 | ||
| RSD, % | 6.00 | |||
|
| ||||
| 100 | 42 | 59.71 | 41.08 | 97.8 |
| 100 | 42 | 59.69 | 39.39 | 93.8 |
| 100 | 42 | 58.34 | 39.18 | 93.3 |
| 100 | 42 | 62.47 | 43.42 | 103.4 |
| 100 | 42 | 59.31 | 39.75 | 94.7 |
| 100 | 42 | 59.25 | 39.53 | 94.1 |
| 100 | 42 | 60.55 | 41.49 | 98.8 |
| 100 | 42 | 60.89 | 41.99 | 100.0 |
| 100 | 42 | 60.89 | 42.93 | 102.2 |
| 100 | 42 | 60.58 | 41.56 | 99.0 |
| 100 | 42 | 60.89 | 41.42 | 98.6 |
| 100 | 42 | 61.19 | 41.94 | 99.8 |
| Mean | 41.14 | 98.0 | ||
| SD | 1.40 | 3.33 | ||
| RSD, % | 3.40 | |||
Measured (spiked) minus 90% value.
With the exception of a few results, recoveries were between 90 and 110% for tomato ketchup (Table 7), orange juice (Table 8), and tomato paste (Table 9).
The analysis of three carbonated beverages is shown in Table 10 and revealed that citric acid was added during production. The spiking level was set at about 50 and 150% of this naturally occurring level. Mean recoveries varied between 96.3 and 104.5%, whereas results for individual test portions were between 93.1 and 106.8%. Precision of repeatability was between 0.31 and 1.76%.
Table 10.
Results of naturally incurred and spiked carbonated beverages; naturally incurred energy drink, orange lemonade, and flavored water were spiked at about 50 and 150% of their naturally occurring content; n = 6 test portions were analyzed
| Non-spiked | Spike 4 g/L |
Spike 12 g/L |
|||||
|---|---|---|---|---|---|---|---|
| g/L | g/L | Difference, g/L | Rec., % | g/L | Difference, g/L | Rec., % | |
| Energy drink, dilution 1:20 | 7.35 | 11.62 | 4.21 | 105.2 | 19.66 | 12.25 | 102.1 |
| 7.33 | 11.55 | 4.15 | 103.7 | 19.52 | 12.11 | 100.9 | |
| 7.31 | 11.68 | 4.27 | 106.8 | 19.52 | 12.11 | 100.9 | |
| 7.46 | 11.57 | 4.17 | 104.1 | 19.58 | 12.17 | 101.4 | |
| 7.59 | 11.55 | 4.14 | 103.6 | 19.46 | 12.05 | 100.4 | |
| 7.40 | 11.55 | 4.15 | 103.6 | 19.57 | 12.16 | 101.4 | |
| Mean | 7.41 | 11.59 | 4.18 | 104.5 | 19.55 | 12.14 | 101.2 |
| SD | 0.11 | 0.05 | 0.05 | 0.07 | 0.07 | ||
| RSD, % | 1.42 | 0.44 | 1.22 | 0.34 | 0.55 | ||
| Non-spiked | Spike 0.8 g/L |
Spike 2.4 g/L |
|||||
|---|---|---|---|---|---|---|---|
| g/L | g/L | Difference, g/L | Rec., % | g/L | Difference, g/L | Rec., % | |
| Orange lemonade, dilution 1:5 | 1.456 | 2.229 | 0.745 | 93.1 | 3.819 | 2.335 | 97.3 |
| 1.489 | 2.254 | 0.770 | 96.2 | 4.005 | 2.521 | 105.0 | |
| 1.478 | 2.241 | 0.757 | 94.6 | 3.954 | 2.470 | 102.9 | |
| 1.487 | 2.264 | 0.780 | 97.4 | 4.009 | 2.525 | 105.2 | |
| 1.480 | 2.267 | 0.783 | 97.9 | 3.969 | 2.485 | 103.6 | |
| 1.515 | 2.301 | 0.817 | 102.1 | 3.939 | 2.455 | 102.3 | |
| Mean | 1.48 | 2.26 | 0.78 | 96.9 | 3.95 | 2.47 | 102.7 |
| SD | 0.02 | 0.02 | 0.02 | 0.07 | 0.07 | ||
| RSD, % | 1.29 | 1.10 | 3.21 | 1.76 | 2.82 | ||
| Non-spiked | Spike 0.36 g/L |
Spike 1.08 g/L |
|||||
|---|---|---|---|---|---|---|---|
| g/L | g/L | Difference, g/L | Rec., % | g/L | Difference, g/L | Rec., % | |
| Flavored water, dilution 1:2 | 0.722 | 1.070 | 0.346 | 96.0 | 1.794 | 1.070 | 99.1 |
| 0.738 | 1.067 | 0.343 | 95.2 | 1.786 | 1.062 | 98.3 | |
| 0.716 | 1.070 | 0.346 | 96.0 | 1.780 | 1.056 | 97.7 | |
| 0.715 | 1.070 | 0.346 | 96.1 | 1.792 | 1.068 | 98.9 | |
| 0.715 | 1.082 | 0.357 | 99.3 | 1.783 | 1.059 | 98.0 | |
| 0.738 | 1.068 | 0.344 | 95.5 | 1.790 | 1.066 | 98.7 | |
| Mean | 0.72 | 1.07 | 0.35 | 96.3 | 1.79 | 1.06 | 98.4 |
| SD | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| RSD, % | 1.56 | 0.50 | 1.54 | 0.31 | 0.52 | ||
Precision of Extraction
To characterize the influence of extraction (and the following pipetting into cuvettes), refer to Tables 7–9 for the naturally incurred matrixes tomato ketchup, orange juice, and tomato paste. RSDs are in all cases at or below 4%. If a liquid sample needs to be diluted only, RSD values around 1% can be obtained (Table 10).
Precision of Repeatability
As seen in other enzymatic assays, pipetting of one extract into different cuvettes seems to be the main contributor of imprecision. To characterize the citric acid assay, one test portion from tomato ketchup and paste was pipetted as six replicates into cuvettes and measured. The experiment was repeated on 2 more days. As can be seen in Table 11, RSD values are quite comparable to the one shown in Tables 7–9. The only exemption was one measurement for tomato paste on day 3. In this case, a higher A1 reading was observed, which can be explained, for example, by insufficient mixing of reagents and test solution. For more data on repeatability, see Intermediate Precision.
Table 11.
Results of naturally incurred and spiked matrix; naturally incurred tomato paste and tomato ketchup sample extracts were used
| Day 1 |
Day 2 |
Day 3 |
||||
|---|---|---|---|---|---|---|
| Replicate | Paste, g/kg | Ketchup, g/kg | Paste, g/kg | Ketchup, g/kg | Paste, g/kg | Ketchup, g/kg |
| 1 | 21.13 | 6.11 | 22.04 | 6.76 | 15.67a | 6.24 |
| 2 | 21.93 | 6.75 | 21.71 | 6.56 | 21.93 | 6.18 |
| 3 | 21.27 | 6.46 | 21.65 | 6.83 | 22.30 | 6.84 |
| 4 | 21.59 | 6.35 | 21.49 | 6.13 | 20.33 | 6.59 |
| 5 | 21.60 | 6.34 | 21.69 | 6.94 | 20.78 | 6.13 |
| 6 | 21.00 | 6.38 | 21.27 | 6.70 | 18.58 | 6.44 |
| Mean | 21.42 | 6.40 | 21.64 | 6.66 | 20.78 | 6.40 |
| SD | 0.35 | 0.21 | 0.25 | 0.29 | 1.47 | 0.27 |
| RSD, % | 1.62 | 3.26 | 1.18 | 4.31 | 7.08 | 4.27 |
A1 high (mixing error).
Interlot Precision
To characterize differences between test kit lots, an interlot precision experiment was set up by analyzing two different aqueous solutions and a wine with n = 6 test portions on one day by one person in all three kit lots. This is necessary to show that all lots are produced under routine and comparable conditions. The results are shown in Table 12 and prove that all three lots were comparable over the whole measurement range. All RSD values were at or below 2% with one exception in one TC for one matrix.
Table 12.
Results of two aqueous solutions and a standard wine measured with n = 6 test portions by one person in all three test kit lots on one day
| Lot 1 |
Lot 2 |
Lot 3 |
All lots | ||||
|---|---|---|---|---|---|---|---|
| Sample | Measured, mg/L | Rec., % | Measured, mg/L | Rec., % | Measured, mg/L | Rec., % | Measured, mg/L |
| Aqueous solution 1000 mg/L | 1026 | 102.6 | 984 | 98.4 | 976 | 97.6 | |
| 1024 | 102.4 | 966 | 96.6 | 978 | 97.8 | ||
| 1020 | 102.0 | 990 | 99.0 | 981 | 98.1 | ||
| 1004 | 100.4 | 972 | 97.2 | 1023 | 102.3 | ||
| 1001 | 100.1 | 956 | 95.6 | 1002 | 100.2 | ||
| 1001 | 100.1 | 974 | 97.4 | 984 | 98.4 | ||
| Mean | 1013 | 101.3 | 974 | 97.4 | 991 | 99.1 | 992 |
| RSD, % | 1.18 | 1.24 | 1.87 | 2.15 | |||
| Multi acid low 250 mg/L | 253 | 101.1 | 240 | 96.0 | 245 | 97.9 | |
| 243 | 97.1 | 252 | 100.9 | 245 | 97.9 | ||
| 241 | 96.4 | 258 | 103.3 | 243 | 97.4 | ||
| 248 | 99.2 | 249 | 99.5 | 246 | 98.3 | ||
| 240 | 95.9 | 249 | 99.5 | 249 | 99.5 | ||
| 242 | 96.9 | 258 | 103.1 | 241 | 96.3 | ||
| Mean | 244 | 97.8 | 251 | 100.4 | 245 | 97.9 | 247 |
| RSD, % | 2.03 | 2.73 | 1.07 | 2.32 | |||
| Wine (standard) #1081608 (orange) 1005 mg/L | 942 | 93.8 | 958 | 95.3 | 948 | 94.3 | |
| 968 | 96.3 | 986 | 98.1 | 988 | 98.3 | ||
| 959 | 95.4 | 949 | 94.4 | 964 | 95.9 | ||
| 964 | 95.9 | 986 | 98.1 | 984 | 97.9 | ||
| 978 | 97.3 | 969 | 96.4 | 956 | 95.2 | ||
| 981 | 97.6 | 983 | 97.8 | 972 | 96.7 | ||
| Mean | 965 | 96.0 | 972 | 96.7 | 969 | 96.4 | 969 |
| RSD, % | 1.45 | 1.64 | 1.61 | 1.50 | |||
Intermediate Precision
To get an idea about intermediate or laboratory-internal reproducibility, one test kit lot was tested on two different days using three different photometers by three laboratory technicians in a laboratory (Table 13). In contrast to former validation studies, the use of three different test kit lots was omitted because it was shown several times before that the parameter “lot” does not contribute very much to the overall variation of results in the case of enzymatic analysis.
Table 13.
Characterization of laboratory-internal reproducibility (intermediate precision) by three analysts extracting or diluting the materials and analyzed them on two different days with three extracts per day and analyzing each extract in two cuvettes
| FAPAS soft drink | NIST cranberry juice | Wine A | Wine B | Ketchup | Orange juice | ||||
|---|---|---|---|---|---|---|---|---|---|
| Analyst | Day | Extract | Cuvette | 2.87 g/L | 3.221 g/L | 1.005 g/L | 0.457 g/L | NA, g/La | NA, g/L |
| 1 | 1 | 1 | 1 | 2.778 | 3.194 | 1.058 | 0.449 | 5.871 | 6.477 |
| 1 | 1 | 1 | 2 | 2.902 | 3.293 | 1.035 | 0.456 | 6.252 | 6.786 |
| 1 | 1 | 2 | 1 | 2.793 | 3.288 | 1.014 | 0.442 | 6.176 | 6.743 |
| 1 | 1 | 2 | 2 | 2.834 | 3.355 | 0.941 | 0.491 | 6.525 | 7.140 |
| 1 | 1 | 3 | 1 | 2.722 | 3.357 | 0.968 | 0.471 | 6.011 | 6.736 |
| 1 | 1 | 3 | 2 | 2.779 | 3.435 | 0.948 | 0.470 | 6.618 | 7.009 |
| 1 | 2 | 4 | 1 | 2.847 | 3.378 | 0.982 | 0.498 | 5.914 | 6.812 |
| 1 | 2 | 4 | 2 | 2.897 | 3.372 | 1.033 | 0.460 | 6.322 | 6.733 |
| 1 | 2 | 5 | 1 | 2.889 | 3.434 | 0.953 | 0.479 | 6.174 | 7.022 |
| 1 | 2 | 5 | 2 | 2.825 | 3.476 | 0.990 | 0.513 | 6.644 | 6.914 |
| 1 | 2 | 6 | 1 | 2.877 | 3.292 | 1.005 | 0.465 | 6.037 | 6.961 |
| 1 | 2 | 6 | 2 | 2.867 | 3.472 | 1.015 | 0.456 | 6.673 | 6.932 |
| 2 | 1 | 1 | 1 | 2.825 | 3.281 | 0.979 | 0.463 | 6.427 | 6.749 |
| 2 | 1 | 1 | 2 | 2.787 | 3.510 | 0.990 | 0.472 | 6.681 | 6.278 |
| 2 | 1 | 2 | 1 | 2.778 | 3.353 | 0.986 | 0.457 | 6.849 | 6.711 |
| 2 | 1 | 2 | 2 | 2.758 | 3.473 | 1.003 | 0.476 | 6.242 | 6.388 |
| 2 | 1 | 3 | 1 | 2.813 | 3.496 | 0.984 | 0.473 | 6.852 | 6.228 |
| 2 | 1 | 3 | 2 | 2.749 | 3.565 | 0.979 | 0.473 | 6.813 | 6.224 |
| 2 | 2 | 4 | 1 | 2.799 | 3.307 | 0.954 | 0.461 | 6.227 | 6.244 |
| 2 | 2 | 4 | 2 | 2.776 | 3.353 | 0.963 | 0.456 | 6.233 | 6.034 |
| 2 | 2 | 5 | 1 | 2.748 | 3.319 | 0.964 | 0.488 | 6.387 | 6.167 |
| 2 | 2 | 5 | 2 | 2.717 | 3.336 | 0.987 | 0.463 | 5.845 | 5.986 |
| 2 | 2 | 6 | 1 | 2.752 | 3.340 | 0.955 | 0.479 | 6.473 | 6.001 |
| 2 | 2 | 6 | 2 | 2.691 | 3.501 | 0.994 | 0.470 | 6.219 | 6.197 |
| 3 | 1 | 1 | 1 | 2.892 | 3.329 | 0.973 | 0.467 | 5.443 | 6.766 |
| 3 | 1 | 1 | 2 | 2.826 | 3.406 | 0.945 | 0.465 | 6.016 | 7.196 |
| 3 | 1 | 2 | 1 | 2.868 | 3.483 | 1.127 | 0.486 | 5.899 | 7.153 |
| 3 | 1 | 2 | 2 | 2.892 | 3.567 | 0.930 | 0.459 | 6.016 | 7.167 |
| 3 | 1 | 3 | 1 | 2.793 | 3.491 | 0.994 | 0.474 | 5.887 | 7.083 |
| 3 | 1 | 3 | 2 | 2.852 | 3.491 | 1.005 | 0.476 | 6.260 | 7.053 |
| 3 | 2 | 4 | 1 | 2.889 | 3.363 | 0.985 | 0.460 | 6.532 | 6.128 |
| 3 | 2 | 4 | 2 | 2.811 | 3.430 | 0.967 | 0.457 | 7.109 | 6.379 |
| 3 | 2 | 5 | 1 | 2.875 | 3.541 | 0.940 | 0.469 | 6.763 | 6.336 |
| 3 | 2 | 5 | 2 | 2.870 | 3.473 | 0.957 | 0.457 | 7.130 | 6.440 |
| 3 | 2 | 6 | 1 | 2.898 | 3.467 | 0.989 | 0.466 | 7.064 | 6.612 |
| 3 | 2 | 6 | 2 | 2.889 | 3.544 | 0.956 | 0.466 | 7.180 | 6.489 |
| Mean, g/L | 2.821 | 3.410 | 0.985 | 0.469 | 6.382 | 6.619 | |||
| SD, g/L | 0.060 | 0.094 | 0.038 | 0.014 | 0.420 | 0.376 | |||
| RSD, % | 2.11 | 2.75 | 3.81 | 2.98 | 6.58 | 5.67 | |||
NA = Not applicable.
The measurement was made with two certified reference materials (FAPAS Soft Drink and NIST Cranberry Juice), two standard wines (Deutsche Weinanalytiker), and two native matrixes from local retailers (tomato ketchup and orange juice). All test samples were extracted by each analyst with n = 3 on each of the two days and analyzed in two cuvettes per extract. Each analyst made the experiment on different days within a period of 3 weeks.
As can be seen in Table 13, the analysis of all originally “clear” matrixes (soft drink, cranberry juice, wines) resulted in an overall RSD of around 3%, with the exception of wine A with an RSD of <4%, which can be attributed to the use of the undiluted wine with a high concentration of citric acid. Tomato ketchup had to be extracted before measurement. This explains the quite high RSD of about 6.5% because several handling steps had to be performed before measurement. Orange juice had to be centrifuged before measurement due to a high content of pulp in the juice. For this matrix, there was a clear difference for analyst 3 when comparing day 1 and day 2.
This experiment was specially designed together with an AOAC statistical expert (Paul Wehling, ChemStats Consulting, Minneapolis, MN, USA) to calculate repeatability, intermediate precision, and the contribution of each type of precision (analyst, day, extraction, and cuvette) by a nested ANOVA design. Table 14 shows the results for repeatability s(r) and intermediate precision s(i) together with their relative measures given in percentage (RSD). Except for ketchup and orange juice, both RSD values are below 4%. Since both performance characteristics are quite close together for each of these four matrixes, it can be concluded that sample preparation such as extraction or centrifugation repeatability is the main driver of total precision (see also Table 15 for more explanations). Ketchup showed higher values for both types of precision, which is attributed mainly to the fact that the extraction is much more complicated than that for orange juice, which is centrifugation and dilution only. The other matrixes had to be diluted only. The quite high value for intermediate precision in the case of orange juice is driven mainly by analyst 3 and the differences between both days of measurement as already mentioned above (see Table 13).
Table 14.
Characterization of intermediate and repeatability precision from the nested analysis of variance
| Performance characteristic | FAPAS soft drink | NIST cranberry juice | Wine A | Wine B | Ketchup | Orange juice |
|---|---|---|---|---|---|---|
| 2.87 g/L | 3.221 g/L | 1.005 g/L | 0.457 g/L | NAa | NA | |
| Mean, g/L | 2.821 | 3.410 | 0.985 | 0.469 | 6.382 | 6.619 |
| sr, g/L | 0.040 | 0.084 | 0.039 | 0.014 | 0.297 | 0.178 |
| RSDr, % | 1.41 | 2.46 | 3.95 | 3.02 | 4.66 | 2.68 |
| si, g/L | 0.065 | 0.097 | 0.039 | 0.015 | 0.539 | 0.411 |
| RSDi, % | 2.32 | 2.85 | 3.94 | 3.17 | 8.45 | 6.22 |
NA = Not applicable.
Table 15.
Characterization of contribution of each variance component (analyst, day, extract, and cuvette) to total precision within one laboratory
| Contributor to total precision | FAPAS soft drink | NIST cranberry juice | Wine A | Wine B | Ketchup | Orange juice |
|---|---|---|---|---|---|---|
| 2.87 g/L % | 3.221 g/L % | 1.005 g/L % | 0.457 g/L % | NAa % | NA % | |
| Analyst | 45.9 | 15.7 | 0.0 | 0.0 | 0.0 | 29.2 |
| Day | 17.0 | 10.0 | 0.0 | 9.7 | 69.6 | 52.2 |
| Extract | 2.9 | 20.6 | 0.0 | 0.0 | 0.0 | 1.2 |
| Residual (cuvette) | 34.2 | 53.7 | 100.0 | 90.3 | 30.4 | 17.4 |
NA = Not applicable.
Table 15 shows that the highest contribution to total precision within a laboratory is depending on the matrix type. For cranberry juice and wines, it is obvious that the pipetting step into the cuvette has the highest contribution to the total precision. The FAPAS material, which is also an easy matrix, seems to be different from the other easy matrixes. In this case, the intermediate precision (2.32%) is already very “high” (see Tables 13 and 14); therefore, small differences due to the analyst may end up in a high percentage of contribution. Tomato ketchup and orange juice had to be extracted/centrifuged before measurement, and since this step was done every day of measurement, it was to be expected that the day has the highest contribution to total precision.
As a take-home message, it can be concluded that the pipetting skills of the analyst will mainly drive the variation of results in the case of citric acid measurement.
Ruggedness Study
These experiments were undertaken to show the influence of variable parameters on test kit results. These parameters are known to be subject of variation during use of the test kit. The parameters tested for their ruggedness were incubation temperature (18 °C, 25 °C, 37 °C; corresponds to 64°F, 77°F, 99°F) and incubation times for A1 (3 min) and for A2 (5, 10, 15, 20 min). Table 16 shows the results of these experiments. To achieve recoveries of 100% ± 5%, the following incubation times are needed: A1 after 3 min and A2 after 15 min for incubation temperatures between 20 and 37 °C (68–99°F).
Table 16.
Ruggedness study at incubation temperatures of 18 to 37 °C (64–99°F) with different incubation times for A2 and a fixed time of 3 min for A1. Samples were aqueous solutions (Solution A, B, C), the commercial Enzytec Multi-acid standard low (E8460), and a wine
| Time A1 = 3 min |
||||||||
|---|---|---|---|---|---|---|---|---|
| 18°C |
25°C |
37°C |
||||||
| Time (A2) | Sample | Target, g/L | g/L | Rec., % | g/L | Rec., % | g/L | Rec., % |
| 5 min | Solution A | 0.100 | 0.090 | 90 | 0.101 | 101 | 0.093 | 93 |
| 0.100 | 0.090 | 90 | 0.101 | 101 | 0.097 | 97 | ||
| Solution B | 0.500 | 0.436 | 87 | 0.477 | 95 | 0.487 | 97 | |
| 0.500 | 0.440 | 88 | 0.471 | 94 | 0.492 | 98 | ||
| Solution C | 1.000 | 0.761 | 76 | 0.852 | 85 | 0.945 | 94 | |
| 1.000 | 0.758 | 76 | 0.860 | 86 | 0.950 | 95 | ||
| Multiacid Std low | 0.249 | 0.234 | 94 | 0.245 | 99 | 0.243 | 97 | |
| 0.249 | 0.225 | 91 | 0.247 | 99 | 0.241 | 97 | ||
| Wine | 0.486 | 0.383 | 79 | 0.419 | 86 | 0.438 | 90 | |
| 0.486 | 0.365 | 75 | 0.415 | 85 | 0.450 | 93 | ||
| 10 min | Solution A | 0.100 | 0.094 | 94 | 0.102 | 102 | 0.098 | 98 |
| 0.100 | 0.099 | 99 | 0.102 | 102 | 0.099 | 99 | ||
| Solution B | 0.500 | 0.493 | 99 | 0.510 | 102 | 0.499 | 100 | |
| 0.500 | 0.496 | 99 | 0.504 | 101 | 0.499 | 100 | ||
| Solution C | 1.000 | 0.972 | 97 | 1.009 | 101 | 0.987 | 99 | |
| 1.000 | 0.973 | 97 | 1.008 | 101 | 0.991 | 99 | ||
| Multiacid Std low | 0.249 | 0.251 | 101 | 0.256 | 103 | 0.247 | 99 | |
| 0.249 | 0.245 | 98 | 0.256 | 103 | 0.247 | 99 | ||
| Wine | 0.486 | 0.470 | 97 | 0.486 | 100 | 0.480 | 99 | |
| 0.486 | 0.458 | 94 | 0.482 | 99 | 0.485 | 100 | ||
| 15 min | Solution A | 0.100 | 0.094 | 94 | 0.105 | 105 | 0.094 | 94 |
| 0.100 | 0.099 | 99 | 0.099 | 99 | 0.100 | 100 | ||
| Solution B | 0.500 | 0.496 | 99 | 0.513 | 103 | 0.496 | 99 | |
| 0.500 | 0.499 | 100 | 0.503 | 101 | 0.498 | 100 | ||
| Solution C | 1.000 | 0.991 | 99 | 1.016 | 102 | 0.985 | 99 | |
| 1.000 | 0.992 | 99 | 1.011 | 101 | 0.990 | 99 | ||
| Multiacid Std low | 0.249 | 0.252 | 101 | 0.258 | 104 | 0.247 | 99 | |
| 0.249 | 0.246 | 99 | 0.257 | 103 | 0.247 | 99 | ||
| Wine | 0.486 | 0.485 | 100 | 0.495 | 102 | 0.481 | 99 | |
| 0.486 | 0.475 | 98 | 0.488 | 100 | 0.488 | 100 | ||
| 20 min | Solution A | 0.100 | 0.093 | 93 | — | — | 0.094 | 94 |
| 0.100 | 0.099 | 99 | — | — | 0.097 | 97 | ||
| Solution B | 0.500 | 0.496 | 99 | — | — | 0.494 | 99 | |
| 0.500 | 0.499 | 100 | — | — | 0.496 | 99 | ||
| Solution C | 1.000 | 0.987 | 99 | — | — | 0.983 | 98 | |
| 1.000 | 0.993 | 99 | — | — | 0.987 | 99 | ||
| Multiacid Std low | 0.249 | 0.252 | 101 | — | — | 0.244 | 98 | |
| 0.249 | 0.246 | 99 | — | — | 0.244 | 98 | ||
| Wine | 0.486 | 0.488 | 100 | — | — | 0.481 | 99 | |
| 0.486 | 0.477 | 98 | — | — | 0.487 | 100 | ||
Dilutability
Dilutability is characterized to check whether a high concentrated test sample can be measured correctly when diluted with deionized water within the measurement range. For the determination, one test sample per matrix was used either directly (orange juice) or after spiking of the (extracted) matrix. Dilution was done with deionized water to result in concentrations within or outside the measurement range. Each diluted extract was analyzed with two replicates per run.
As can be seen in Figures 4–7, a test solution diluted to a measured value of 1.2 g/L is already in the linear range of the system, which perfectly matches the characterization of linearity using aqueous solutions.
Figure 4.
Results for dilutability of an orange juice (citric acid concentration was around 8 g/L before extraction); equation for regression was calculated without the data marked as open circle; n = 2 per dilution were analyzed in two independent runs.
Figure 5.
Results for dilutability of an extracted tomato ketchup (citric acid concentration was around 6.5 g/L before extraction); equation for regression was calculated without the data marked as open circles; n = 2 per dilution were analyzed in two independent runs.
Figure 6.
Results for dilutability of an extracted tomato paste (citric acid concentration was around 20 g/L before extraction); equation for regression was calculated without the data marked as open circles; n = 2 per dilution were analyzed in two independent runs.
Figure 7.
Results for dilutability of a spiked wine sample (citric acid concentration was around 0.3 g/L before spiking to 2.0 g/L); equation for regression was calculated without the data marked as open circles; n = 2 per dilution were analyzed in two independent runs.
Real-Time Stability Study
Three different samples were tested in three test kit lots over a period of 24 months by six different persons. As can be seen in Table 17, there is no trend toward increasing or decreasing values. The RSD values are at 2.5% or below.
Table 17.
Characterization of real-time stability of the enzymatic system over a maximum period of 24 months using three lots stored at 2–8 °C; three different control samples were analyzed in duplicate by six different analysts
| Citric acid solution | Multi-acid solution | Standard wine | ||||||
|---|---|---|---|---|---|---|---|---|
| Lot | Month | Analyst | Target: 1000 mg/L | Target: 250 mg/L | Target: 1005 mg/L | |||
| 3 | 0 | 1 | 981 | 1002 | 254 | 250 | 955 | 943 |
| 0 | 2 | 993 | 1006 | 260 | 246 | 996 | 994 | |
| 8 | 3 | 1035 | 1040 | 262 | 255 | 976 | 975 | |
| 10 | 4 | 1037 | 1021 | 263 | 253 | 996 | 1014 | |
| 12 | 5 | 1022 | 1019 | 257 | 255 | 978 | 975 | |
| 15 | 6 | 1027 | 1027 | 256 | 253 | 984 | 984 | |
| 18 | 6 | 1020 | 1015 | 259 | 258 | 974 | 973 | |
| 21 | 6 | 1001 | 997 | 257 | 254 | 939 | 941 | |
| 24 | 6 | 1003 | 1008 | 252 | 250 | 942 | 946 | |
| 2 | 0 | 1 | 1000 | 1006 | 250 | 258 | 990 | 1012 |
| 0 | 2 | 1011 | 1015 | 249 | 254 | 1022 | 992 | |
| 8 | 3 | 1034 | 1032 | 260 | 259 | 961 | 987 | |
| 10 | 4 | 998 | 1010 | 246 | 251 | 989 | 1016 | |
| 12 | 5 | 1019 | 1009 | 254 | 252 | 977 | 995 | |
| 15 | 6 | 1019 | 1027 | 251 | 257 | 1003 | 988 | |
| 18 | 6 | 1015 | 1021 | 257 | 256 | 985 | 963 | |
| 21 | 6 | 994 | 990 | 253 | 247 | 935 | 932 | |
| 24 | 6 | 1005 | 1005 | 254 | 250 | 936 | 934 | |
| 1 | 0 | 1 | 1000 | 1012 | 256 | 249 | 1012 | 991 |
| 0 | 2 | 1023 | 1017 | 261 | 253 | 996 | 995 | |
| 8 | 3 | 1049 | 1053 | 260 | 264 | 971 | 992 | |
| 10 | 4 | 1034 | 1040 | 254 | 259 | 1000 | 1006 | |
| 12 | 5 | 1025 | 1016 | 259 | 260 | 988 | 986 | |
| 15 | 6 | 1022 | 1023 | 260 | 261 | 992 | 965 | |
| 18 | 6 | 1014 | 1015 | 258 | 252 | 975 | 974 | |
| 21 | 6 | 1006 | 1002 | 262 | 250 | 942 | 958 | |
| 24 | 6 | 1006 | 1009 | 251 | 249 | 959 | 933 | |
| Mean | 1015 | 255 | 977 | |||||
| SD | 14.9 | 4.5 | 24.4 | |||||
| RSD, % | 1.47 | 1.78 | 2.50 | |||||
Stability Study on Transportation
To investigate the influence of harsh transport conditions, a simulated transport stability was performed. The conditions that were simulated included shaking and temperature changes. All components of lot TC3 were placed on a horizontal shaker at room temperature and agitated for 6 h; 400 rpm were used at the beginning and changed later on to 150 rpm. Afterwards, the components were refrigerated for 18 h at 2–8 °C (36–46°F), followed by 7 h at room temperature on a horizontal shaker (150 rpm). Components were incubated at 37 °C (99°F) for 18 h and after cooling down to room temperature measured, on the same day, with aqueous citric acid standards and a wine as usual. Afterwards, the stressed components were stored at 2–8 °C (36–46°F) and were rechecked after 12 and 24 months in the QC department. As can be seen in Table 18, the simulation of transportation shows now no effects on functionality and quality of the components directly after performing the simulation (t-test with a P-value of 0.424). Values after 12 and 24 months are not shown but revealed no tendency toward higher or lower values.
Table 18.
Results after transport stability for one test kit lot using three different control solutions and a wine sample
| Reference | Transport | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Target | Measured |
|||||
| Sample | Dilution | Citric acid, mg/L | Citric acid, mg/L | Rec., % | Citric acid, mg/L | Rec., % |
| Citric acid solution | 1 | 1000 | 995 | 99.5 | 1004 | 100.4 |
| Citric acid solution | 1 | 1000 | 992 | 99.2 | 1008 | 100.8 |
| Multi acid low | 1 | 250 | 261 | 104.3 | 258 | 103.1 |
| Multi acid low | 1 | 250 | 245 | 98.2 | 260 | 104.1 |
| Wine (standard) orange | 2 | 1005 | 1021 | 101.6 | 983 | 97.8 |
| Wine (standard) orange | 2 | 1005 | 992 | 98.7 | 1025 | 102.0 |
| Citric acid solution | 1 | 500 | 503 | 100.6 | 502 | 100.4 |
| Citric acid solution | 1 | 500 | 483 | 96.7 | 506 | 101.2 |
Stability Study on Freezing
To simulate an unintended freezing of the test kit components, the whole test kits of lot TC 3 was frozen for 24 h at –20 °C (–4°F). Afterwards, the components were allowed to warm up to room temperature and were frozen again at –20 °C (–4°F). After 24 h, the components were thawed and after warming up to room temperature finally measured with aqueous citric acid standards and a wine test sample against unstressed components. As shown in Table 19, the repeated freezing overnight did not affect the functionality of the test system significantly (t-test P-value is 0.353).
Table 19.
Results for stability after freezing of one test kit lot using aqueous citric acid solutions and a wine sample
| Reference | Freezing | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Target | Measured |
|||||
| Sample | Dilution | Citric acid, mg/L | Citric acid, mg/L | Rec., % | Citric acid, mg/L | Rec., % |
| Citric acid solution | 1 | 1000 | 995 | 99.5 | 999 | 99.9 |
| Citric acid solution | 1 | 1000 | 992 | 99.2 | 1008 | 100.8 |
| Multi acid low | 1 | 250 | 261 | 104.3 | 254 | 101.6 |
| Multi acid low | 1 | 250 | 245 | 98.2 | 232 | 92.9 |
| Wine (standard) orange | 2 | 1005 | 1021 | 101.6 | 997 | 99.2 |
| Wine (standard) orange | 2 | 1005 | 992 | 98.7 | 977 | 97.2 |
| Citric acid solution | 1 | 500 | 503 | 100.6 | 496 | 99.2 |
| Citric acid solution | 1 | 500 | 483 | 96.7 | 493 | 98.6 |
Stability Study of Already Opened and Reused Components
To simulate the behavior when test kit components are opened and used several times, bottles R1 and R2 of lot TC 2 were opened and closed again after a pipetting step. These test kits were given to the QC department for further regular check together with the regular stability test for unopened test kits (real-time stability). Results are not shown, but after 24 months of using these opened test kits, there was no tendency toward higher or lower values of the control solutions.
Automation on a Pictus 500 Spectrophotometric Analyzer
Comments on validation parameters independent on automation.—Side reactivity to other related organic acids or other substances and interfering substances will not be characterized on the automated analyzer because there is no known effect that an automated process will change the reactivity toward these substances. The pipetting environment within a closed automate is much more regulated than the normal laboratory environment (including the analyst). For measurement, all reagents are cooled at 8 ± 2 °C (42–50°F), while the reaction zone where the analysis takes place is tempered to 37 °C (99°F). This ensures a quick enzymatic reaction and highly reproducible results. Therefore, the characterization of incubation times and temperatures was not repeated. Incubation at 37 °C (99°F) and the necessary incubation times are described for the 4 mL cuvette manual application (Table 16). There is also no practical reason to analyze test kit components that were tested for their stabilities against transport, freezing, and short-term storage at 37 °C. As already mentioned, the Pictus 500 can automatically change between the three applications in case the concentration is below or above the basic measurement range. Therefore, it was decided not to characterize each application for an LOD.
-
LOQ.—The lower end of the measurement range is the limit of quantification, where acceptable recovery and precision are met. Our internal requirements are a recovery higher than 95% and an RSD equal or lower than 10%. For each of the three applications, aqueous solutions with different concentrations of citric acid were analyzed at least five times. The concentration was calculated from the calibration of the system.
Figure 8 shows the results for the basic application with a test volume of 10 µL, which is—despite a factor of 10 in volumes—the identical ratio of test volume to reagents as the manual format with a test volume of 100, 2000 µL reagent 1 and 500 µL reagent 2. The automated analyzer has an LOQ of 40 mg/L using the criteria described before. The manual format exerted a calculated LOQ of about 20 mg/L, but the precision at this point is still not sufficient. Data from a precision plot gave the same LOQ of 40 mg/L for the manual format.
Since citric acid is often present at quite high concentrations in food such as fruit juices, the automated high range application with a low test volume of 2 µL was introduced to analyze these matrixes without dilution prior to measurement. As can be seen in Figure 9, the LOQ for this application is 0.5 g/L. This application was not investigated for the manual format because this would require test volumes of 20 µL, which is challenging for untrained analysts. The Pictus 500 shows RSD values at or below 5% for a test volume of 2 µL.
In case trace analysis of citric acid is necessary, the sensitive range application with a test volume of 100 µL was investigated for its LOQ (Figure 10). An LOQ of 8 mg/L can be claimed for this application where recovery and precision requirements were met.
-
Linearity.—The most important parameter for an automated application is the linear range because in the case of enzymatic analysis, the analyte is often always present in the test sample and its proper quantification only depends on the proper choice of test volume and calibration. For each of the three applications, the optimal linear measurement range was characterized.
Figure 11 shows that the upper measurement range is 1.2 g/L for a test volume of 10 µL. It should be noted that aged test kits will always reach this high concentration. Therefore, the claimed upper measurement range is 1 g/L.
For the high range application with a test volume of 2 µL, the upper measurement range is 5 g/L (Figure 12). This is a factor of five compared to the basic application and perfectly fits to the increased test volume of 10 µL.
For the sensitive application with a test volume of 100 µL, the upper measurement range is 100 mg/L (Figure 13). It is always recommended to include control solutions at the upper range to check for linearity.
On-board and calibration stability.—Data on these important characteristics will be provided for the Final Action decision because these experiments are ongoing. Customers use the assay in many different ways over a longer period (e.g., some will let the reagents in the automate until they are empty). Others will store them after each working day in the refrigerator to prevent deterioration. The setup of such an experiment is therefore challenging.
Precision and recovery.—The precision of the automated pipetting was characterized for all three applications. Due to the different measurement ranges of each application, different test samples had to be used. In most cases, aqueous solutions were used because the characterization of different matrixes was already done during the validation of the manual application. To check for trueness, one standard wine was applied for the high range application.
Figure 8.
Confirmation of LOQ for the basic application with 10 µL test volume; RSD values are given as open triangles and recoveries as closed circles.
Figure 9.
Confirmation of LOQ for the high range application with 2 µL test volume; RSD values are given as open triangles and recoveries as closed circles.
Figure 10.
Confirmation of LOQ for the sensitive range application with 100 µL test volume; RSD values are given as open triangles and recoveries as closed circles.
Figure 11.
Characterization of linearity for the basic application with 10 µL test volume; RSD values are given as open triangles and recoveries as closed circles; data given as open circles were not included in linear regression.
Figure 12.
Characterization of linearity for the high range application with 2 µL test volume; RSD values are given as open triangles and recoveries as closed circles; data shown as open circles were not included in linear regression.
Figure 13.
Characterization of linearity for the sensitive application with 100 µL test volume; RSD values are given as open triangles and recoveries as closed circles; data shown as open circles were not included in linear regression.
Table 20 shows the results for the basic range application with a test volume of 10 µL. As expected for automated pipetting, RSDs at or below 1% were obtained for concentrations between 0.25 and 1.0 g/L. The validity of the two-point calibration (0 and 1.0 g/L) was also checked with these three solutions. Recoveries ranged between 98.8 and 101.2% and were thus clearly within specifications.
Table 20.
Characterization of precision for the basic range (10 µL sample volume) application using three aqueous solutions
| Aqueous solution |
|||
|---|---|---|---|
| Target Replicate | 1.000, g/L | 0.250, g/L | 0.500, g/L |
| 1 | 1.006 | 0.250 | 0.491 |
| 2 | 1.026 | 0.255 | 0.496 |
| 3 | 1.017 | 0.252 | 0.494 |
| 4 | 1.015 | 0.258 | 0.495 |
| 5 | 1.008 | 0.251 | 0.493 |
| 6 | 1.009 | 0.249 | 0.500 |
| 7 | 1.007 | 0.251 | 0.495 |
| 8 | 1.010 | 0.251 | 0.491 |
| 9 | 1.013 | 0.249 | 0.494 |
| 10 | 1.008 | 0.250 | 0.492 |
| Mean | 1.012 | 0.252 | 0.494 |
| SD | 0.0061 | 0.0028 | 0.0027 |
| RSD, % | 0.60 | 1.13 | 0.54 |
| Recovery, % | 101.2 | 100.6 | 98.8 |
Table 21 shows the results for the high range application with a test volume of 2 µL. As expected for automated pipetting and the small volume, RSDs at or below 2% were obtained for concentrations between 0.25 and 1.005 g/L. The validity of the two-point calibration (0 and 5.0 g/L) was also checked with the two solutions and the standard wine. Recoveries ranged between 98.4 and 99.1% and were thus clearly within specifications. The standard wine comes with a certificate so that trueness of the system was established for the application with the smallest volume of 2 µL.
Table 21.
Characterization of precision for the high range (2 µL sample volume) application using two aqueous solutions and a standard wine sample
| Aqueous solution |
Wine | ||
|---|---|---|---|
| Target Replicate | 1.000, g/L | 0.250, g/L | 1.005, g/L |
| 1 | 1.007 | 0.251 | 0.985 |
| 2 | 0.993 | 0.241 | 0.981 |
| 3 | 0.978 | 0.249 | 1.004 |
| 4 | 0.982 | 0.248 | 0.973 |
| 5 | 0.994 | 0.247 | 0.980 |
| 6 | 0.984 | 0.249 | 0.987 |
| 7 | 0.994 | 0.245 | 1.001 |
| 8 | 0.970 | 0.258 | 0.983 |
| 9 | 0.992 | 0.244 | 0.994 |
| 10 | 0.993 | 0.244 | 1.001 |
| Mean | 0.989 | 0.248 | 0.989 |
| SD | 0.0104 | 0.0048 | 0.0104 |
| RSD, % | 1.05 | 1.93 | 1.05 |
| Recovery | 98.9 | 99.1 | 98.4 |
Table 22 shows the results for the sensitive range application with a test volume of 100 µL. For this application, a four-point calibration (0, 11.1, 33.3, and 100 mg/L) was established and validated. RSD values were at 1.5% or lower for concentrations of 50 or 100 mg/L. The lowest concentration of 25 mg/L showed a higher variation of about 5%, which is still acceptable because citric acid normally occurs at higher concentrations in food. The validity of the four-point calibration was also checked with the three solutions. Recoveries ranged between 95.2 and 100.0% and were thus within specifications.
Table 22.
Characterization of precision for the sensitive range application (100 µL sample volume) using three aqueous solutions
| Aqueous solution |
|||
|---|---|---|---|
| Target Replicate | 0.100, g/L | 0.025, g/L | 0.050, g/L |
| 1 | 0.100 | 0.024 | 0.048 |
| 2 | 0.100 | 0.025 | 0.047 |
| 3 | 0.100 | 0.024 | 0.047 |
| 4 | 0.100 | 0.023 | 0.048 |
| 5 | 0.102 | 0.021 | 0.048 |
| 6 | 0.100 | 0.025 | 0.049 |
| 7 | 0.100 | 0.024 | 0.048 |
| 8 | 0.098 | 0.023 | 0.048 |
| 9 | 0.100 | 0.024 | 0.047 |
| 10 | 0.100 | 0.025 | 0.049 |
| Mean | 0.100 | 0.024 | 0.048 |
| SD | 0.0009 | 0.0012 | 0.0007 |
| RSD, % | 0.94 | 5.17 | 1.54 |
| Recovery, % | 100.0 | 95.2 | 95.8 |
Conclusion
In summary, the data of the in-house validation study proved that the performance claims for food and beverages such as wines, juices, and tomato products are fulfilled. The method is robust and accurate for both applications (manual and automated). The test kit Enzytec Liquid Citric acid was approved as AOAC Official Method of Analysis for quantification of citric acid in the claimed matrixes.
Acknowledgments
We would like to thank Patricia Meinhardt (R-Bioharm, Inc., Washington, MO, USA) and Paul Wehling (ChemStats, Minneapolis, MN, USA). From R-Biopharm AG (Darmstadt, Germany), the following persons are greatly acknowledged for their practical contributions: Rebecca Ziegler, Rebecca Kredel, Tina Dubois, Berthold Wagner, and Ute Maelzer-Funk. We would like to thank the entire AOAC Expert Review Panel and especially Ruth Ivory for their excellent contributions.
Contributor Information
Markus Lacorn, R-Biopharm AG, An der Neuen Bergstr. 17, 64297 Darmstadt, Germany.
Thomas Hektor, R-Biopharm AG, An der Neuen Bergstr. 17, 64297 Darmstadt, Germany.
Conflict of Interest
Both authors were employed by the company manufacturing the test kit while conducting the study.
Funding
Funding was paid by the manufacturer who also pays salaries for Lacorn and Hektor.
References
- 1. OIV (2015) International Code of Oenological Practices—3. Wines: 3.3.8 Treatment with Citric Acid (16/70). https://www.oiv.int/standards/international-code-of-oenological-practices/part-ii-oenological-treatments-and-practices/wines/treatment-with-citric-acid (accessed July 22, 2024) [Google Scholar]
- 2. Zacchia M., Preisig P. (2010) J. Nephrol. 23 Suppl 16, S49–56 [PubMed] [Google Scholar]
- 3. Kline E.E., Treat E.G., Averna T.A., Davis M.S., Smith A.Y., Sillerud L.O. (2006) J. Urol. 176, 2274–2279. doi: 10.1366/11-06546 [DOI] [PubMed] [Google Scholar]
- 4. Lacorn M., Hektor T. (2018) J. AOAC Int. 101, 1101–1111. doi: 10.5740/jaoacint.17-0466 [DOI] [PubMed] [Google Scholar]
- 5. Lacorn M., Heßler-Noll M., Wehling P., Hektor T. (2023) J. AOAC Int. 106, 341–347. doi: 10.1093/jaoacint/qsac145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Souci S.W., Fachmann W., Kraut H. (2016) Food Composition and Nutrition Tables, 8th revised and completed edition, MedPharm
- 7. Linsinger T. ERM Application Note no. 1, https://crm.jrc.ec.europa.eu/graphics/cms_docs/erm1_english.pdf (accessed June 6, 2024)