Abstract
Background/purpose
Dual-cure resin-cements are used for various dental restorations. However, whether the curing modes of these resin-cements influence gingival inflammation remains unclear. Hence, herein, we evaluated the effects of dual-cure resin-cement curing modes on gingival cytotoxicity and inflammatory responses.
Materials and methods
Specimens were prepared using two dual-cure resin-cements—RelyX Unicem 2 (RU) and G-CEM ONE (GO)—by light-cure or self-cure modes. Degree of conversion (DC) and monomer elution of the resin-cements were measured using Fourier-transform infrared spectroscopy and high-performance liquid chromatography, respectively. Human gingival fibroblasts (GFs) and macrophages were cultured on resin-cements, and inflammatory cytokine levels, intracellular reactive oxygen species (ROS) generation, and mitogen-activated protein (MAP) kinase activation were evaluated.
Results
Light-cured (LC) resin-cements exhibited significantly higher DC and lower monomer elution than did self-cured (SC) resin-cements. Triethyleneglycol dimethacrylate (TEGDMA) and urethane dimethacrylate (UDMA) were substantially eluted from RU and GO, respectively. Neither LC resin-cement exhibited cytotoxicity and enhanced pro-inflammatory cytokine expression in GFs and macrophages. However, both SC resin-cements significantly decreased cell numbers and promoted cellular inflammatory responses. SC generated higher intracellular ROS levels compared to that seen with LC, and different patterns of MAP kinase activation were observed between SC–RU and SC–GO.
Conclusion
Compared with LC dual-cure resin-cements, SC dual-cure resin-cements show stronger cytotoxicity and elicit greater inflammatory responses in gingival cells owing to residual monomers (e.g., TEGDMA and UDMA) by activating MAP kinases in GFs and macrophages. Clinicians should ensure adequate light irradiation during prosthesis cementation and make efforts to remove the excess cement.
Keywords: Curing modes, Cytotoxicity, Dual-cure resin-cements, Gingival tissue, Inflammatory responses
Introduction
The use of translucent materials such as lithium disilicate and yttria-stabilized tetragonal zirconia polycrystal for indirect restoration is increasing with the development of dental materials.1,2 Nevertheless, metal and opaque zirconia are still being used to prevent prosthesis fracture and mask the color of dichromatic abutment teeth.3 Dual-cure resin-cements are widely used for various indirect restorations because they can be cured using the self-cure mode even when light access is severely restricted owing to restorative dental materials.4 However, self-cured (SC) dual-cure resin-cements exhibit a lower degree of conversion and inferior mechanical properties than those seen with light-cured (LC) dual-cure resin-cements.5,6 SC resin-cements demonstrate higher residual monomer elution than do LC resin-cements.7 Resin monomers, such as triethylene glycol dimethacrylate (TEGDMA) and 2-hydroxyethyl methacrylate (HEMA), may induce cytotoxicity8 and inflammatory responses by producing intracellular reactive oxygen species (ROS) and activating mitogen-activated protein (MAP) kinases.9, 10, 11 Therefore, eluted monomers from dual-cure resin-cements might elicit adverse effects in the oral tissue.
After cementation, the removal of excess resin-cement around the prosthesis poses challenges. Cement remnants are detected in 56% of patients with prostheses.12 An in vitro study reported cement remnants around crowns, despite cleaning procedures.13 Moreover, clinical complications associated with cement remnants have been reported. When peri-implant tissue was compared between screw-retained and cement-retained prostheses, increased inflammatory reactions were detected in the cement-retained prosthesis group,14 along with higher levels of the pro-inflammatory cytokine interleukin (IL)-1β in the peri-implant sulcular fluid.15 Cement-retained crowns exhibit more pathological bone resorption than do screw-retained restorations.16 Meanwhile, an in vitro study demonstrated the elution of resin monomers from the marginal cement of crown restorations,17 indicating that both excessive resin-cements and marginal resin-cements of the prostheses can affect gingival tissue. However, whether curing modes of dual-cure resin-cements influence gingival inflammation are poorly understood. In this study, we investigated the effects of dual-cure resin-cement curing modes on inflammatory responses of gingival tissue cells.
Materials and methods
Preparation of discs using dual-cure resin-cement
Two commercially available dual-cure resin-cements, RelyX Unicem 2 Automix (RU: A2; 3M, Saint Paul, MN, USA) and G-CEM ONE EM (GO: A2; GC Corporation, Tokyo, Japan) (Table 1), were used in this study. The resin-cement was placed in a stainless-steel mold with a diameter and thickness of 13 and 1 mm, respectively, to fabricate discs. In the LC group, the resin-cements were irradiated for 10 s from above using a handheld LED light-curing unit (G-Light Prima II; GC) in the 1200-mW cm−2 mode. For the SC group, the resin-cements were incubated at 37 °C for 10 min and then polished and cleaned in distilled water ultrasonically.
Table 1.
Composition of dual-cure resin-cements.
| Material | Composition |
|---|---|
| RelyX Unicem2 (RU) Shade: A2 3M, Saint Paul, MN, USA |
[Paste A] Triethyleneglycol dimethacrylate, 2-hydroxypropane-1,3-diyl-bis(2-methylprop-2-enoate) and 3-hydroxypropane-1,2-diyl-bis(2-methylprop-2-enoate) and diphosphorus pentoxide, sodium persulfate, copper (II) acetate monohydrate, tert-butyl peroxy-3,5,5-trimethylhexanoate, glass filler, and initiator. [Paste B] Substituted dimethacrylate, 1,12-dodecane dimethacrylate, 2-Propenoic acid, 2-methyl-, [(3methoxypropyl)imino]di-2,1-ethanediyl ester, 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-phenyl-1(phenylmethyl)-, calcium salt, calcium hydroxide, titanium dioxide, glass filler, and initiator. |
| G-CEM ONE (GO) Shade: A2 GC, Tokyo, Japan |
[Paste A] Urethane dimethacrylate (UDMA), 6-ter-butyl-2,4-xylenol, diphenyl (2,4,6-trimethylbenzoyl) phosphine oxide, glass filler, and initiator. [Paste B] UDMA, 10-methacryloyloxydecyl dihydrogen phosphate, 6-ter-butyl-2,4-xylenol, α,α-dimethylbenzoyl hydroperoxide, glass filler, and initiator. |
Measurement of degree of conversion
Fourier-transform infrared spectroscopy (FT/IR-6700; JASCO, Tokyo, Japan) was used to measure the degree of conversion (DC) of the specimens. Attenuated total reflectance spectra were measured at 400–4000 cm−1 and the 64 scans were performed at 4 cm−1. Infrared absorption peaks of aliphatic and aromatic bonds were detected at 1637 and 1610 cm−1. The ratio (R) between the aliphatic bonds of the methacrylate functional group and the aromatic bonds of bisphenol was calculated, and the DC ratio was calculated using the following equation:
| DC (%) = 100 (1 − Rpolym/Runpolym), |
where Runpolym is R of the unpolymerized cement and Rpolym is R of the polymerized cement.
Measurement of vickers hardness
A hardness tester (HM-102; Mitsutoyo, Kanagawa, Japan) was used to measure Vickers hardness of the specimens. The surface of the specimens was pressed using an indenter at 0.20 kgf for 15 s. The following equation was used for calculating Vickers hardness:
| HV = 1.8544F/d2 |
where HV is Vickers hardness, F is load force (kgf), and d is the diagonal length of the indentation (mm).
Measurement of resin monomer release
The specimens were immersed in 5 mL of distilled water for 7 days. A high-performance liquid chromatography instrument (Chromaster; Hitachi High-Tech, Tokyo, Japan) was used to measure resin monomer release from the specimens. The column used was Inert Sustain AQ-C18 (GL Science, Tokyo, Japan), with an internal diameter of 4.5 mm, length of 250 mm, and filler-particle size of 3 μm. The mobile phase (75% methanol and 25% distilled water) was introduced at a flow rate of 1 mL/min; the detection wavelength was 206 nm. Loops with a capacity of 5 μL were injected. The standard solutions of TEGDMA and urethane dimethacrylate (UDMA) in acetonitrile were used to calculate the monomer concentration.
Culture of human gingival fibroblasts
Human gingival fibroblasts (GFs) were isolated from a healthy patient after obtaining consent. The GFs were expanded in Dulbecco's modified Eagle's medium (DMEM; Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and 100 U penicillin/0.1 mg/mL streptomycin. The GFs were seeded onto the prepared discs in a 12-well plate (Thermo Fisher Scientific) at a density of 1 × 105 cells/well and cultured in the culture medium at 37 °C in a humidified incubator.
Culture of THP-1 cells
The human monocytic cell line, THP-1 (JCRB0112.1; Japanese Collection of Research Bioresources Cell Bank, Osaka, Japan), was expanded in DMEM supplemented with 10% FBS and 100 U penicillin/0.1 mg/mL streptomycin. THP-1 cells were seeded onto the discs in a 12-well plate at a density of 1 × 105 cells/well, and 100 nM phorbol 12-myristate 13-acetate (PMA; Wako Chemicals, Osaka, Japan) was added into the culture medium to induce macrophage differentiation.
Real time RT–PCR
Total RNA was extracted from the cells using the RNeasy kit (QIAGEN, Germantown, MD, USA), and RNA quantification was performed using a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). After DNase I treatment (Thermo Fisher Scientific), cDNA was synthesized from the RNA using a reverse transcriptase (Super Script VILO; Thermo Fisher Scientific). SYBR green-based PCR (Toyobo, Osaka, Japan) or TaqMan probe-based PCR (Thermo Fisher Scientific) was performed to determine mRNA expression, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the internal control. The primers are listed in Table 2. The 2–ΔΔCt method was used to analyze gene expression.
Table 2.
Primers used for RT–PCR analyses.
| Gene | Primers (Fw, forward; Rv, reverse) | Product size (bp) | Accession number |
|---|---|---|---|
| Interleukin (IL)-1β | Fw: 5′-TGGAGCAACAAGTGGTGT-3′ | 157 | NM_000576.3 |
| Rv: 5′-TTGGGATCTACACTCTCCAGC-3′ | |||
| IL-6 | Fw: 5′-TCAATGAGGAGACTTGCCTG-3′ | 157 | NM_001371096.1 |
| Rv: 5′-GATGAGTTGTCATGTCCTGC-3′ | |||
| Prostaglandin E synthethase 2 | Fw: 5′-TCCAGTACCAAAATCGTATTGCT-3′ | 370 | NM_000963.4 |
| Rv: 5′-AGTGCTTCCAACTCTGCAGACAT-3′ | |||
| Tumor necrosis factor α | Fw: 5′-GAGGCCAAGCCCTGGTATG-3′ | 91 | NM_000594.4 |
| Rv: 5′-CGGGCCGATTGATCTCAGC-3′ | |||
| Matrix metalloproteinases 2 (MMP2) | Fw: 5′-TGAGCTATGGACCTTGGGAGAA-3′ | 60 | NM_001302510.2 |
| Rv: 5′-CCATCGGCGTTCCCATAC-3′ | |||
| MMP9 | Fw: 5′-GGACGATGCCTGCAACGT-3′ | 64 | NM_004994.3 |
| Rv: 5′-CAAATACAGCTGGTTCCCAATCT-3′ | |||
| Nitric oxide synthase 2 | Fw: 5′-CAGCGGGATGACTTTCCAA-3′ | 75 | NM_000625.4 |
| Rv: 5′-AGGCAAGATTTGGACCTGCA-3′ | |||
| Glyceraldehyde 3-phosphate dehydrogenase | Fw: 5′-AATCCCATCACCATCTTCCA-3′ | 82 | NM_001357943.2 |
| Rv: 5′-TGGACTCCACGACGTACTCA-3′ |
Cell proliferation assay
After cell culture, 10% water-soluble tetrazolium 1 (WST-1) reagent (Roche, Basel, Switzerland) was added to the culture medium and incubated at 37 °C for 90 min. Absorbance was then measured using an absorbance reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
DNA quantification
Total cellular DNA was measured using a DNA quantification kit (COSMO BIO Co., Ltd., Tokyo, Japan). After cell lysis, Hoechst 33258 was added into the cell lysate, and fluorescence intensity was measured using a microplate reader (Promega Co., Madison, WI, USA) with an excitation peak at 365 nm and an emission peak at 410–460 nm.
Intracellular reactive oxygen species levels evaluation
After cell culture, the culture medium was replaced with phosphate-buffered saline containing 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (Thermo Fisher Scientific), and the cells were incubated at 37 °C for 15 min. Fluorescence intensity was measured using a microplate reader (Molecular Devices, San Jose, CA, USA) with an excitation peak at 490 nm and an emission peak at 510 nm. The intracellular ROS level was normalized to the total DNA concentration.
Western blotting
The cells were lysed using lysis buffer composed of 50 mM Tris 7.5, 120 mM NaCl, and 0.5% NP-40 in the presence of phosphatase inhibitor (Sigma–Aldrich, Saint Louis, MO, USA). Protein concentration was measured using a protein assay reagent (Bio-Rad Laboratories, Inc.). After electro-transfer of protein to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.), the membrane was blocked with 5% skim milk (BD Biosciences, Franklin Lakes, NJ, USA) and incubated at 4 °C overnight with the primary antibody (Cell Signaling Technology, Danvers, MA, USA), namely anti-phospho-extracellular signal-regulated kinase 1 and 2 (ERK1/2) antibody, anti-phospho-p38 antibody, anti-phospho-c-Jun N-terminal kinase (JNK) antibody, anti-ERK1/2 antibody, anti-p38 antibody, anti-JNK antibody, or anti-β-actin antibody. Subsequently, the membrane was washed with tris-buffered saline with tween 20 (10 mM Tris–HCl, 100 mM NaCl, and 0.1% Tween) and incubated with secondary antibodies (Santa Cruz Biotechnology, Dallas, TX, USA) at 25 °C for 1 h. A horseradish peroxidase enzyme (Merck Millipore, Burlington, MA, USA) was used to visualize the bands.
Statistical analysis
Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey's multiple comparison tests. Statistical significance was set at P < 0.05. Sample sizes are described in the figure legends.
Results
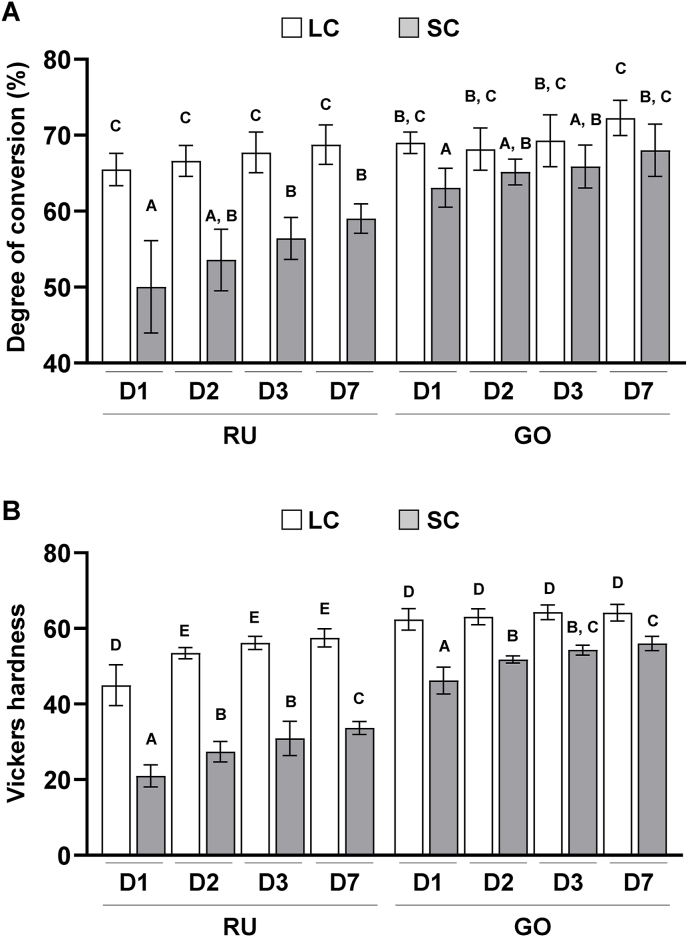
Both SC–RU and SC–GO exhibited lower DC than the LC counterparts. The difference between SC and LC resin-cements was larger in the RU group than in the GO group (Fig. 1A). Consistent with the DC, both SC–RU and SC–GO exhibited significantly lower Vickers hardness values than the LC counterparts (P < 0.05). In addition, SC–RU showed a lower Vickers hardness than SC–GO (Fig. 1B). These results suggest that SC dual-cure resin-cements exhibit lower polymerization than LC dual-cure resin-cements.
Figure 1.
Effects of dual-cure resin-cement [RelyX Unicem 2 (RU) and G-CEM ONE (GO)] curing mode on the degree of conversion (DC) and mechanical properties of dual-cure resin-cements. (A) DC of light-cured (LC) or self-cured (SC) resin-cements determined using Fourier-transform infrared spectroscopy with a universal attenuated total reflectance (n = 6). (B) Vickers hardness of LC or SC resin-cements measured with a microhardness tester (n = 10). Data were analyzed using analysis of variance (ANOVA) and Tukey's multiple comparison test, and P < 0.05 was considered significant. Data are presented as the mean ± standard deviation (SD), with different letters indicating statistically significant differences between multiple groups. D1, day 1; D2, day 2; D3, day 3; D7, day 7.
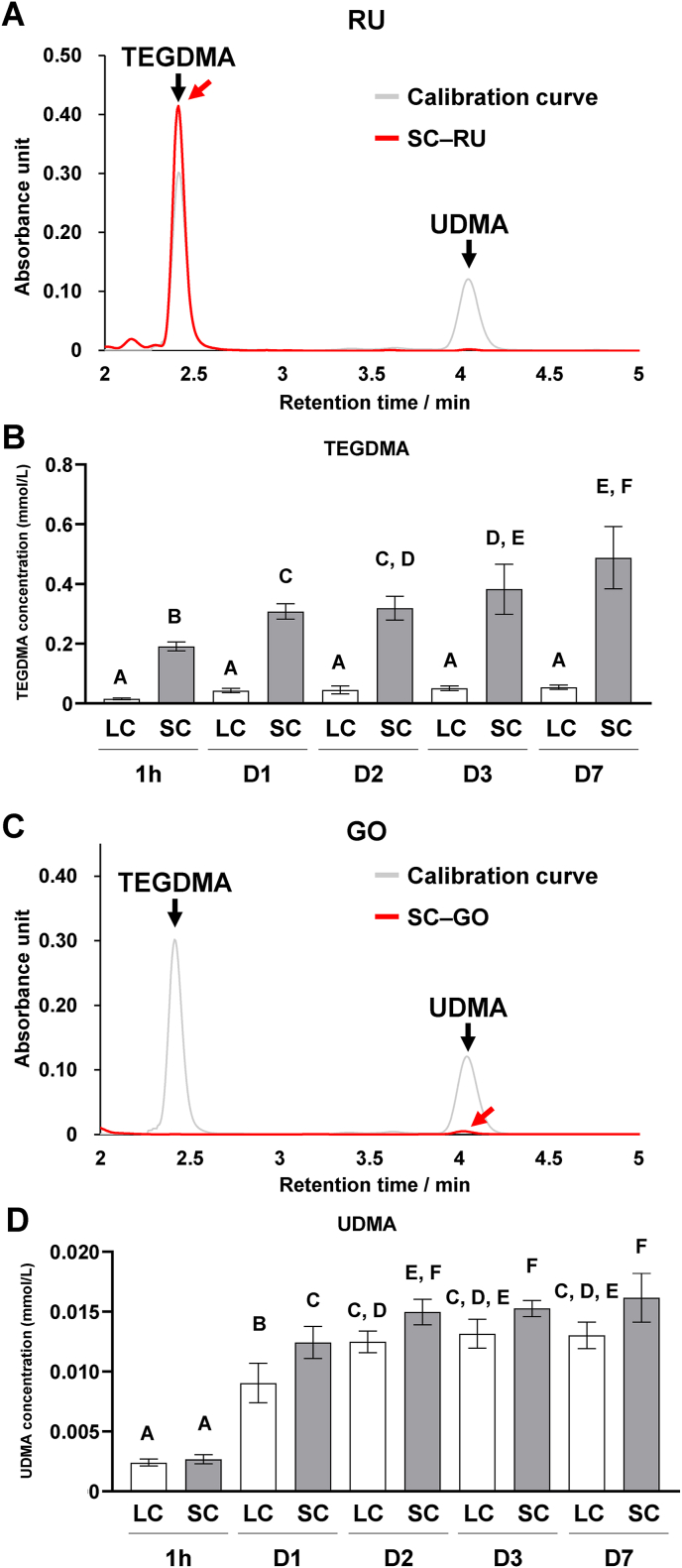
TEGDMA was identified in the supernatant of RU following immersion in water; UDMA was absent (Fig. 2A). Significantly higher concentrations of released residual TEGDMA were noted in the SC group than that in the LC group (P < 0.05). TEGDMA concentrations gradually rose from day 1–7 in the SC group (Fig. 2B). By contrast, GO revealed a small UDMA peak in the supernatant post water immersion, with no detection of TEGDMA (Fig. 2C). The concentration of released residual UDMA increased progressively from 1 h to 7 days in both the LC and SC groups. Notably, the UDMA concentration in the SC group was higher than that in the LC group, and the difference was significant from days 1–7 (P < 0.05; Fig. 2D).
Figure 2.
Effects of dual-cure resin-cement [RelyX Unicem 2 (RU) and G-CEM ONE (GO)] curing mode on resin monomer release from dual-cure resin-cements. (A) Representative chromatograms of triethylene glycol dimethacrylate (TEGDMA) and urethane dimethacrylate (UDMA) standard solutions and the supernatant of self-cured RU (SC–RU) immersed in water on day 1. (B) Released TEGDMA concentrations in the supernatant of light-cured (LC) or SC–RU immersed in water (n = 7). (C) Representative chromatograms of TEGDMA and UDMA standard solutions and the supernatant of SC–GO immersed in water on day 1. (D) Released UDMA concentrations in the supernatant of LC or SC–GO immersed in water (n = 7). Red arrows indicate detected monomer peaks. Data were analyzed using analysis of variance (ANOVA) and Tukey's multiple comparison test, and P < 0.05 was considered significant. Data are presented as the mean ± standard deviation (SD), with different letters indicating statistically significant differences between multiple groups. 1h, 1 h; D1, day 1; D2, day 2; D3, day 3; D7, day 7.
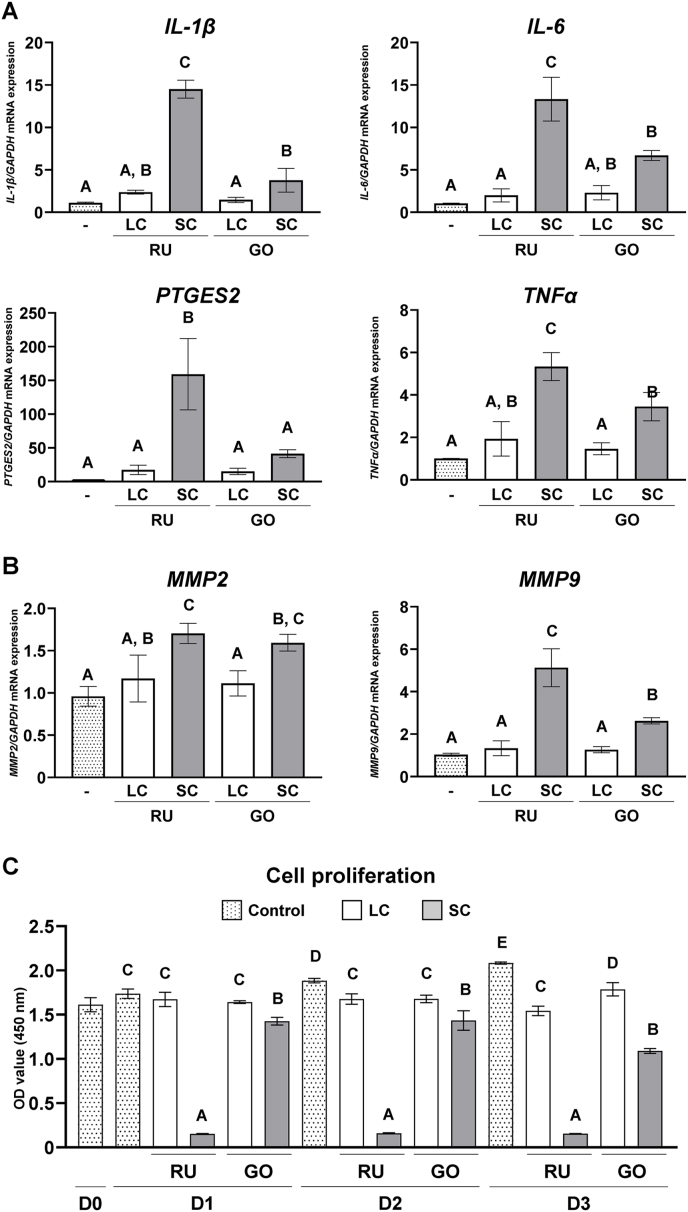
Expression of pro-inflammatory cytokines, such as IL-1β, IL-6, prostaglandin E synthetase 2 (PTGES2), and tumor necrosis factor α (TNFα), matrix metalloproteinase 2 (MMP2), and MMP9 was upregulated in GFs by both SC resin-cements compared with the no-resin-cements and LC resin-cement groups. The SC–RU group exhibited significantly higher expression of these genes, except for MMP2, than did the SC–GO group (P < 0.05; Fig. 3A and B). Moreover, the number of GFs cultured on SC–RU was significantly lower than that in the other groups (P < 0.05), and those cultured on SC–GO decreased from day 1–3 (Fig. 3C). These results indicate that SC resin-cements induce the inflammatory response in GFs and exhibit higher cytotoxicity for GFs compared with the LC resin-cements.
Figure 3.
Effects of dual-cure resin-cement [RelyX Unicem 2 (RU) and G-CEM ONE (GO)] curing mode on gingival fibroblasts (GFs). Gene expression of (A) pro-inflammatory cytokines interleukin (IL)-1β, IL-6, prostaglandin E synthase 2 (PTGES2), tumor necrosis factor α (TNFα), and (B) matrix metalloproteinase 2 (MMP2) and MMP9 relative to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in GFs cultured on light-cured (LC) or self-cured (SC) resin-cements on day 1 (n = 3). (C) Water-soluble tetrazolium 1 (WST-1)-based evaluation of GF cell proliferation cultured on LC or SC resin-cements (n = 3). Data were analyzed using analysis of variance (ANOVA) and Tukey's multiple comparison test, and P < 0.05 was considered significant. Data are presented as the mean ± standard deviation (SD), with different letters indicating statistically significant differences between multiple groups. D0, day 0; D1, day 1; D2, day 2; D3, day 3.
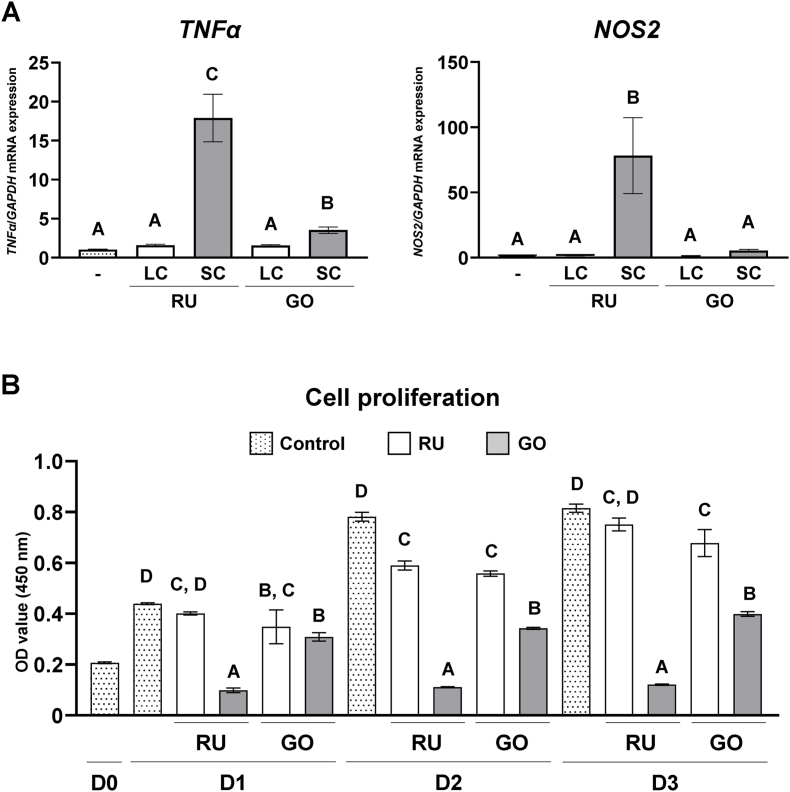
Both SC resin-cements upregulated the expression of pro-inflammatory cytokines TNFα and nitric oxide synthase 2 (NOS2) in THP-1-derived macrophages compared with their LC counterparts. Notably, SC–RU displayed significantly higher expression levels than did SC–GO (P < 0.05; Fig. 4A). Furthermore, the numbers of THP-1-derived macrophages cultured on SC–RU and SC–GO were significantly lower than those cultured on the LC counterparts (P < 0.05), and the number of cells was significantly lower in the SC–RU group than in the SC–GO group (P < 0.05; Fig. 4B). These results suggest that SC resin-cements exhibit higher pro-inflammatory response and cytotoxicity in macrophages compared with LC resin-cements.
Figure 4.
Effects of dual-cure resin-cement [RelyX Unicem 2 (RU) and G-CEM ONE (GO)] curing mode on macrophages. Gene expression of (A) pro-inflammatory cytokines tumor necrosis factor α (TNFα) and nitric oxide synthase 2 (NOS2) relative to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in macrophages cultured on light-cured (LC) or self-cured (SC) resin-cements on day 1 (n = 3). (B) Water-soluble tetrazolium 1 (WST-1)-based evaluation of cell proliferation of macrophages cultured on LC or SC resin-cements (n = 3). Data were analyzed using analysis of variance (ANOVA) and Tukey's multiple comparison test, and P < 0.05 was considered significant. Data are presented as the mean ± standard deviation (SD), with different letters indicating statistically significant differences between multiple groups. D0, day 0; D1, day 1; D2, day 2; D3, day 3.
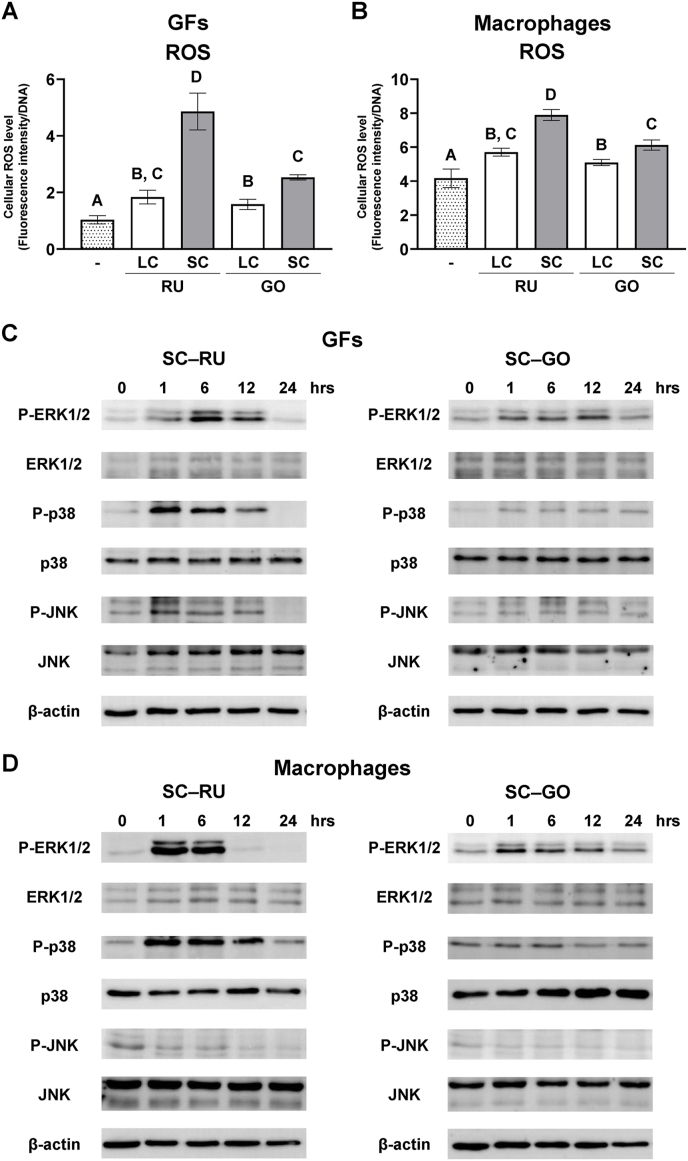
Both SC resin-cements significantly elevated intracellular ROS levels in GFs and THP-1-derived macrophages compared with the LC resin-cements (P < 0.05). The ROS level was significantly higher in the SC–RU group than in the SC–GO group (P < 0.05; Fig. 5A and B). MAP activation was also evaluated using SC–RU and SC–GO. In the RU group, ERK1/2, p38, and JNK in the GFs were phosphorylated at 1 h, with ERK1/2 phosphorylation peaking at 6 h (Fig. 5C). GO also induced ERK1/2 phosphorylation at 1 h in the GFs; however, the degree of phosphorylation was weak compared with that in the RU group. p38 and JNK were slightly phosphorylated in GFs treated with GO at 1 and 6 h, respectively (Fig. 5D). In THP-1-derived macrophages, ERK1/2 and p38 were strongly phosphorylated at 1 h by RU, whereas JNK was not phosphorylated (Fig. 5E). GO induced the phosphorylation of ERK1/2 at 1 h in THP-1-derived macrophages, while P38 and JNK were not phosphorylated (Fig. 5F). These results indicate that SC resin-cements induce higher levels of intracellular ROS in GFs and macrophages, resulting in MAP activation; however, patterns of MAP kinase activation vary between the products.
Figure 5.
Effects of dual-cure resin-cement [RelyX Unicem 2 (RU) and G-CEM ONE (GO)] curing mode on intracellular reactive oxygen species (ROS) generation and mitogen-activated protein (MAP) kinase activation of gingival fibroblasts (GFs) and macrophages. Intracellular ROS levels of (A) GFs and (B) macrophages cultured on light-cured (LC) or self-cured (SC) resin-cements measured and normalized to DNA concentrations at 1 h (n = 3). Activation of MAP kinases in (C) GFs and (D) macrophages cultured on SC resin-cements. Data were analyzed using analysis of variance (ANOVA) and Tukey's multiple comparison test, and P < 0.05 was considered significant. Data are presented as the mean ± standard deviation (SD), with different letters indicating statistically significant differences between multiple groups. hrs, hours; P-ERK1/2, phosphorylated extracellular signal-regulated kinase 1 and 2; P-p38, phosphorylated p38; P-JNK, phosphorylated c-Jun N-terminal kinase (JNK).
Discussion
The influence of curing mode on the physiological and mechanical properties of dual-cure resin-cements and the release of residual resin monomers have been intensively studied.5, 6, 7,18 However, the effects of dual-cure resin-cement curing modes on gingival tissue remain unknown. In this study, LC dual-cure resin-cements showed less cytotoxicity and inflammatory reactions in GFs and macrophages, when compared with their SC counterparts.
Resin monomers induce inflammatory responses via ROS generation.10 Bisphenol A-glycidyl-methacrylate promotes the production of prostaglandin E2 and cyclooxygenase-2 in human dental pulp cells via intracellular ROS production.9 Moreover, exposure to HEMA enhances intracellular ROS levels and the release of IL-1β in RAW264.7 macrophages.19 Similarly, TEGDMA activates inflammatory responses in RAW264.7 cells through ROS generation.20 Consistently, this study demonstrated that SC–RU, with TEGDMA elution, enhances pro-inflammatory cytokine expression in GFs and macrophages through ROS generation. UDMA induces ROS generation21 and activates innate immunity.22 We also demonstrated that SC–GO with UDMA release upregulates pro-inflammatory cytokine expression in GFs and macrophages through intracellular ROS generation, although the amount of eluted UDMA is relatively small. Thus, resin monomers eluted from SC resin-cements induce strong inflammatory effects on innate immunity. Polarization of macrophages into pro-inflammatory M1 and anti-inflammatory M2 macrophages is crucial for tissue inflammatory responses.23,24 In this study, expressions of pro-inflammatory M1 macrophage markers, such as TNFα and NOS2, were lower in the LC resin-cements group than in the SC resin-cements group, indicating that light irradiation for dual-cure resin-cements could modulate the pro-inflammatory state of macrophages. In clinical settings, sufficient light irradiation should be ensured to promote polymerization in marginal and excessive cement, even when prostheses are fabricated using metal or opaque zirconia. Moreover, clinicians should make every effort to remove the excess cement.
Resin monomers activate MAP, including ERK1/2, p38, and JNK.25 Exposure to HEMA or TEGDMA induces phosphorylation of ERK1/2, p38, and JNK in rat salivary gland acinar cells.26 Consistent with previous research, the present study indicated that TEGDMA eluted from RU strongly activates ERK1/2, p38, and JNK in human GFs and macrophages. Furthermore, in the present study, UDMA eluted from GO induced the phosphorylation of ERK1/2, p38, and JNK in human GFs and macrophages, as well as in other resin monomers. To the best of our knowledge, this is the first study to demonstrate that UDMA activates MAP kinases in GFs and macrophages.
The DC of SC resin-cements depends on polymerization via a chemical reaction. Chemically activated polymerization is induced and promoted by redox initiators (e.g., amines, benzoyl peroxide) to generate free radicals.27 Manufacturers have utilized co-initiators (e.g., sodium sulfinate salts) for resin-cements to accelerate redox-based chemical polymerization.28,29 Furthermore, a recent study demonstrated that using thiourethane with low concentrations of p-tolyldiethanolamine and benzoyl peroxide as chemical initiators improves the DC of SC resin-cements.30 Thus, chemicals and co-initiators are essential for the polymerization of SC resin-cements. Here, we demonstrated that SC–GO exhibits significantly more DC than SC–RU, resulting in lower inflammatory effects of GO in gingival tissue cells compared with those in SC–RU. This is attributed to the more efficient progression of chemically activated polymerization of GO compared with that of RU. Although the details of the chemical initiators were not identified for RU and GO, the difference in chemical initiators between these products is implicated in the results of this study.
Commercially available resin-cements contain different resin monomers and chemical initiators. However, we assessed only two commercially available products to evaluate the biological effects of these cements, which is a limitation of this study. Although our results provide valuable insights for understanding inflammatory responses in gingival tissue around prostheses using resin-cements, further studies using multiple resin-cements are warranted.
In this study, although the degree of the inflammation caused by dual-cure resin-cements varied with residual monomer (e.g., TEGDMA and UDMA) content, LC dual-cure resin-cements showed significantly lower cytotoxicity and inflammatory responses of gingiva cells. Therefore, clinicians must ensure adequate light irradiation during prostheses cementation using dual-cure resin-cements and remove any excess cement. Future research should aim to establish, evaluate, and confirm the optimal guidelines for prosthesis cementation.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported in part by Grants-in-Aid for Young Scientists (Start-up: 22K20983, T.K.) and Early-Career Scientists (23K16081, T.K.) from the Japan Society for the Promotion of Science.
Contributor Information
Takeru Kondo, Email: takeru.kondo.a7@tohoku.ac.jp.
Hiroshi Egusa, Email: egu@tohoku.ac.jp.
References
- 1.Mutlu-Sağesen H.L.E., Sağesen E.A., Özcan M. Bibliometric analysis of zirconia publications between 1980 and 2021: global productivity and publication trends. J Prosthodont Res. 2024;68:147–155. doi: 10.2186/jpr.JPR_D_22_00316. [DOI] [PubMed] [Google Scholar]
- 2.Chen T.A., Lu P.Y., Lin P.Y., et al. Effects of ceramic thickness, ceramic translucency, and light transmission on light-cured bulk-fill resin composites as luting cement of lithium disilicate based-ceramics. J Prosthodont Res. 2024;68:255–263. doi: 10.2186/jpr.JPR_D_22_00304. [DOI] [PubMed] [Google Scholar]
- 3.Jo E.H., Huh Y.H., Ko K.H., et al. Effect of different ceramic materials and substructure designs on fracture resistance in anterior restorations. J Prosthet Dent. 2022;127:785–792. doi: 10.1016/j.prosdent.2020.09.056. [DOI] [PubMed] [Google Scholar]
- 4.David-Pérez M., Ramírez-Suárez J.P., Latorre-Correa F., et al. Degree of conversion of resin-cements (light-cured/dual-cured) under different thicknesses of vitreous ceramics: systematic review. J Prosthodont Res. 2022;66:385–394. doi: 10.2186/jpr.JPR_D_20_00090. [DOI] [PubMed] [Google Scholar]
- 5.Aldhafyan M., Silikas N., Watts D.C. Influence of curing modes on thermal stability, hardness development and network integrity of dual-cure resin cements. Dent Mater. 2021;37:1854–1864. doi: 10.1016/j.dental.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Aldhafyan M., Silikas N., Watts D.C. Influence of curing modes on conversion and shrinkage of dual-cure resin-cements. Dent Mater. 2022;38:194–203. doi: 10.1016/j.dental.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Aldhafyan M., Silikas N., Watts D.C. Influence of curing modes on monomer elution, sorption and solubility of dual-cure resin-cements. Dent Mater. 2022;38:978–988. doi: 10.1016/j.dental.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Issa Y., Watts D.C., Brunton P.A., et al. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent Mater. 2004;20:12–20. doi: 10.1016/s0109-5641(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 9.Chang M.C., Lin L.D., Chan C.P., et al. The effect of BisGMA on cyclooxygenase-2 expression, PGE2 production and cytotoxicity via reactive oxygen species- and MEK/ERK-dependent and -independent pathways. Biomaterials. 2009;30:4070–4077. doi: 10.1016/j.biomaterials.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 10.Schmalz G., Krifka S., Schweikl H. Toll-like receptors, LPS, and dental monomers. Adv Dent Res. 2011;23:302–306. doi: 10.1177/0022034511405391. [DOI] [PubMed] [Google Scholar]
- 11.Schweikl H., Spagnuolo G., Schmalz G. Genetic and cellular toxicology of dental resin monomers. J Dent Res. 2006;85:870–877. doi: 10.1177/154405910608501001. [DOI] [PubMed] [Google Scholar]
- 12.Linkevicius T., Puisys A., Vindasiute E., et al. Does residual cement around implant-supported restorations cause peri-implant disease? A retrospective case analysis. Clin Oral Implants Res. 2013;24:1179–1184. doi: 10.1111/j.1600-0501.2012.02570.x. [DOI] [PubMed] [Google Scholar]
- 13.Augusti D., Augusti G., Re D. Undetected excess cement at marginal areas of zirconia crown copings: in vitro analysis of two luting agents and their influence on retention. Int J Prosthodont (IJP) 2020;33:202–211. doi: 10.11607/ijp.6531. [DOI] [PubMed] [Google Scholar]
- 14.Weber H.P., Kim D.M., Ng M.W., et al. Peri-implant soft-tissue health surrounding cement- and screw-retained implant restorations: a multi-center, 3-year prospective study. Clin Oral Implants Res. 2006;17:375–379. doi: 10.1111/j.1600-0501.2005.01232.x. [DOI] [PubMed] [Google Scholar]
- 15.Ali D. Levels of interleukin 1-beta and soluble urokinase plasminogen activation factor in peri-implant sulcular fluid of cement- and screw-retained dental implants. Quintessence Int. 2023;54:452–458. doi: 10.3290/j.qi.b3877567. [DOI] [PubMed] [Google Scholar]
- 16.Ragauskaitė A., Žekonis G., Žilinskas J., et al. The comparison of cement- and screw-retained crowns from technical and biological points of view. Stomatol. 2017;19:44–50. [PubMed] [Google Scholar]
- 17.Putzeys E., Vercruyssen C., Duca R.C., et al. Monomer release from direct and indirect adhesive restorations: a comparative in vitro study. Dent Mater. 2020;36:1275–1281. doi: 10.1016/j.dental.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Yang B., Huang Q., Holmes B., et al. Influence of curing modes on the degree of conversion and mechanical parameters of dual-cured luting agents. J Prosthodont Res. 2020;64:137–144. doi: 10.1016/j.jpor.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Bolling A.K., Solhaug A., Morisbak E., et al. The dental monomer hydroxyethyl methacrylate (HEMA) counteracts lipopolysaccharide-induced IL-1β release—possible role of glutathione. Toxicol Lett. 2017;270:25–33. doi: 10.1016/j.toxlet.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Yang L.C., Chang Y.C., Yeh K.L., et al. Protective effect of Rutin on triethylene glycol dimethacrylate-induced toxicity through the inhibition of caspase activation and reactive oxygen species generation in macrophages. Int J Mol Sci. 2022;23 doi: 10.3390/ijms231911773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang C.Y., Chiang C.Y., Chiang Y.W., et al. Toxic effects of urethane dimethacrylate on macrophages through caspase activation, mitochondrial dysfunction, and reactive oxygen species generation. Polymers. 2020;12:1398. doi: 10.3390/polym12061398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alizadehgharib S., Östberg A.K., Dahlstrand Rudin A., et al. The effects of the dental methacrylates TEGDMA, Bis-GMA, and UDMA on neutrophils in vitro. Clin Exp Dent Res. 2020;6:439–447. doi: 10.1002/cre2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondo T., Yamada M., Egusa H., et al. Innate immune regulation in dental implant osseointegration. J Prosthodont Res. 2024 doi: 10.2186/jpr.JPR_D_23_00198. [DOI] [PubMed] [Google Scholar]
- 24.Kondo T., Otake K., Kakinuma H., et al. Zinc- and fluoride-releasing bioactive glass as a novel bone substitute. J Dent Res. 2024 doi: 10.1177/00220345241231772. [DOI] [PubMed] [Google Scholar]
- 25.Kondo T., Kakinuma H., Fujimura K., et al. Incomplete polymerization of dual-cured resin cement due to attenuated light through zirconia induces inflammatory responses. Int J Mol Sci. 2023;24:9861. doi: 10.3390/ijms24129861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krifka S., Petzel C., Hiller K.A., et al. Resin monomer-induced differential activation of MAP kinases and apoptosis in mouse macrophages and human pulp cells. Biomaterials. 2010;31:2964–2975. doi: 10.1016/j.biomaterials.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Ikemura K., Endo T. Effect on adhesion of new polymerization initiator systems comprising 5-monosubstituted barbituric acids, aromatic sulfinate amides, and tert-butyl peroxymaleic acid in dental adhesive resin. J Appl Polym Sci. 1999;72:1655–1668. [Google Scholar]
- 28.Arrais C.A., Giannini M., Rueggeberg F.A. Effect of sodium sulfinate salts on the polymerization characteristics of dual-cured resin cement systems exposed to attenuated light-activation. J Dent. 2009;37:219–227. doi: 10.1016/j.jdent.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Faria-e-Silva A.L., Moraes R.R., Ogliari F.A., et al. The role of the primer. J Oral Sci. 2009;51:255–259. doi: 10.2334/josnusd.51.255. [DOI] [PubMed] [Google Scholar]
- 30.Faria-E-Silva A.L., Pfeifer C.S. Development of dual-cured resin cements with long working time, high conversion in absence of light and reduced polymerization stress. Dent Mater. 2020;36:293–301. doi: 10.1016/j.dental.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]