Summary
Mutations in the human genes encoding the endothelin ligand-receptor pair EDN3 and EDNRB cause Waardenburg-Shah syndrome (WS4), which includes congenital hearing impairment. The current explanation for auditory dysfunction is defective migration of neural crest-derived melanocytes to the inner ear. We explored the role of endothelin signaling in auditory development in mice using neural crest-specific and placode-specific Ednrb mutation plus related genetic resources. On an outbred strain background, we find a normal representation of melanocytes in hearing-impaired mutant mice. Instead, our results in neural crest-specific Ednrb mutants implicate a previously unrecognized role for glial support of synapse assembly between auditory neurons and cochlear hair cells. Placode-specific Ednrb mutation also caused impaired hearing, resulting from deficient synaptic transmission. Our observations demonstrate the significant influence of genetic modifiers in auditory development, and invoke independent and separable roles for endothelin signaling in the neural crest and placode lineages to create a functional auditory circuitry.
Subject areas: Molecular neuroscience, Cellular neuroscience, Sensory neuroscience
Graphical abstract

Highlights
-
•
Hearing loss is variable in outbred endothelin signaling mutant mice as in humans
-
•
Melanocyte deficiency does not occur in hearing impaired Edn3-Ednrb mutant mice
-
•
Ednrb action in neural crest-derived glia supports auditory synapse formation
-
•
Ednrb in placode derived-auditory sensory neurons supports synaptic transmission
Molecular neuroscience; Cellular neuroscience; Sensory neuroscience
Introduction
The auditory system arises from two distinct ectodermal lineages, placode and neural crest. The placode-derived otic vesicle gives rise to the cochlea including mechanosensory hair cells, supporting cells, and the non-melanocyte populations of the stria vascularis. The prosensory domain of the otic placode gives rise to spiral ganglion (auditory) neurons (SGNs); these are bipolar neurons that extend peripheral axons to the hair cells and also project into an ascending auditory pathway (the dorsal and ventral cochlear nuclei) of the central nervous system.1,2,3 SGNs are specified into two functionally distinct types: type I neurons establish afferent synapses on inner hair cells (IHCs), and type II neurons extend afferent axon branches and synapse on outer hair cells (OHCs).4 The hair cells reside in the cochlear duct, a compartment filled with potassium-enriched endolymph which provides the electrochemical environment that is crucial for hair cell mechanotransduction. The ionic composition (endocochlear potential) of endolymph is maintained by neural crest-derived melanocytes that comprise the intermediate cell layer of the stria vascularis.5 Neural crest also gives rise to glia, including satellite cells that enfold individual SGNs in the spiral ganglia, and Schwann cells that are associated with peripheral auditory nerve fibers.6 In mammals, the final establishment of normal auditory circuitry occurs postnatally (in mice, over the first 2 postnatal weeks). Developmental perturbations that result in defective hair cell mechanotransduction and/or defective mechanosensory circuitry result in congenital sensorineural hearing impairment or deafness, which is a significant human burden.7,8 While the anatomical composition of the normal auditory system is well defined, many of the molecular features that underlie the developmental and maturation events that create auditory functionality or that result in dysfunction are not well studied.
Waardenburg syndrome (WS) is a human congenital disorder characterized by pigmentation abnormalities and sensorineural deafness, and is classified into subtypes based on additional phenotypic components.9 Type 4 Waardenburg syndrome (WS4), also called Waardenburg-Shah syndrome, is distinguished from other WS types because of its association with a congenital gut motility disorder known as Hirschsprung disease (HSCR).10 Mutations in genes encoding endothelin receptor type B (EDNRB) and its ligand endothelin 3 (EDN3) are prominently associated with human WS4A and WS4B, respectively.11 Importantly, the same mutations in EDNRB and EDN3 are also associated with only HSCR without WS pathologies (HSCR-2 and -3, respectively), indicating variable penetrance of auditory and pigmentation phenotypes.
Defective migration of neural crest cells to a variety of sites is believed to account for all pathological features demonstrated in human WS4. HSCR is characterized by a failure of neural crest-derived enteric nervous system progenitors to migrate into the distal colon, which results in an aganglionic segment in the terminal bowel and thereby a failure of colonic peristaltic contraction. Similarly, melanocytes (pigment cells) throughout the body arise from the neural crest and defective migration of melanoblasts explains pigmentation abnormalities in human WS. In previous studies using inbred mouse strain backgrounds, Edn3-Ednrb gene mutations recapitulated human WS and HSCR disease pathologies including aganglionic colon and coat color pigmentation defects.12,13,14,15 Ednrb-deficient mice exhibited an absence of neural crest-derived melanocytes in the stria vascularis (intermediate stria cells) of the cochlea, which like HSCR and coat color alterations could result from a melanocyte migration defect.14,15,16 These melanocytes generate the endocochlear potential and are essential for hearing, so their absence would explain deafness. To date, absence of neural crest-derived intermediate stria cells is accepted as the primary etiology of sensorineural deafness in WS4.
In this study, we utilized the genetically diverse outbred ICR mouse strain background to examine auditory function in Edn3-Ednrb global and conditional mutant mice. Unlike prior mouse studies but as observed in humans, all mutant mice exhibited aganglionic colon (HSCR) whereas hearing impairment was variably penetrant. We make two additional key observations. First, hearing impaired mutant mice on this strain background have a normal contribution of melanocytes to the stria vascularis. This challenges the conclusion that deafness in WS is exclusively the result of stria melanocyte migration deficiency; our results instead point to an additional glial-specific (neural crest) role in mechanosensory synapse formation. Second, we show that there is an independent required role for Edn3-Ednrb signaling within the placode-derived sensory neurons to enable auditory functionality. Here, our results implicate an SGN-specific role in synaptic transmission. Our findings establish a dual lineage origin of congenital hearing loss in Edn3-Ednrb type WS4, force reevaluation of the paradigm for auditory defects in the context of these mutations, and demonstrate the complex genetics that cause Ednrb-deficiency to manifest in auditory function above or below a hearing threshold.
Results
Incomplete penetrance of hearing loss in endothelin signaling mutant mice
Genetic background affects the penetrance and expression of many phenotypes. Variable degrees of pigmentation abnormalities and colonic aganglionosis in Ednrb null rodents are examples of this dynamic.17 Auditory defects, however, have either been present or absent in these Ednrb null rodent models with no indication of variable penetrance.14,15 We performed click-evoked auditory brain response (ABR) on Ednrb- and Edn3-null mutant mice that have been maintained in the genetically diverse outbred ICR background. All homozygous mutants for the global Ednrb or Edn3 alleles died within a few days of postnatal day 21 with chronic constipation from colonic aganglionosis (i.e., HSCR).18 All animals including control littermates were therefore subjected for ABR at postnatal day 18–19, and their hearing (ABR threshold) determined as the lowest intensity at which a representable ABR waveform could be visually identified (Figure S1). All control mice (Table S1) showed thresholds in the range of 50–70 (average 60.24 ± 6.91) decibels sound pressure level (dB SPL) (Figures 1A and 1B). The hearing threshold of control mice on the ICR background improved to 40–60 (average 49.76 ± 1.02) dB SPL in the next few days (Figure S2), indicating that hearing is not fully matured at the age of P18-19. In 41 global Ednrb mutant mice (from 15 litters generated from 8 independent breeding pairs), 17 mutants showed no response even at the highest sound pressure level (>90dB) and 12 mutants showed an ABR threshold at 80 or 90dB. Surprisingly, 12 mutants showed a comparable response to littermate controls (ABR threshold ≤70dB). These were bilateral traits: all of the first 10 mutants tested as hearing-impaired on one ear exhibited impaired hearing on the other ear, and the first 10 of 10 mutants showing normal hearing on one ear also demonstrated normal hearing on the other ear. Overall, 71% of global Ednrb mutant mice exhibited hearing impairment (ABR threshold ≥80dB or no response). Similarly, 61% of global Edn3 null mice (76 mice from 27 litters generated from 14 independent breeding pairs) showed hearing impairment (19 with no response, 27 with ABR threshold ≥80dB, 30 with ABR threshold ≤70dB). Thus, in this outbred background, auditory defects were very prominent but incompletely penetrant, whereas HSCR was fully penetrant. This resembles the presentation of defects in humans with EDN3 or EDNRB gene mutations.8 Because ICR background mice are albino, pigmentation phenotypes could not be scored.
Figure 1.
Incomplete penetrance of hearing impairment in Edn3-Ednrb signaling deficient mice
(A) Compiled representation of the percentage of mice of each genotype that exhibited the indicated ABR threshold. Black bars, genetic controls (see also Table S1); dark gray bars, hearing mutants; light gray bars, hearing impaired mutants (ABR threshold 80-90dB); white bars, non-hearing mutants (NR = no response at the highest stimulus intensity (90dB)).
(B) Table summarizing the number of animals tested and phenotype frequency for global Ednrb, global Edn3, Wnt1Cre/Ednrb and Pax2Cre/Ednrb mutant mice.
(C) Mean DPOAEs (±SEM) from controls (n = 26) and a subset of hearing impaired (ABR threshold≥80dB) Ednrb (n = 8), Edn3 (n = 15), Wnt1Cre/Ednrb (n = 15) and Pax2Cre/Ednrb (n = 13) mutants at L1 intensity level (dB SPL) of the indicated frequency (kHz). L1 is the stimulus level of the first (f1) primary tone (see STAR Methods).
(D) Mean amplitude vs. latency plots for wave I in ABR waveforms at 90dB (left) and 80dB (right) acquired from hearing mutants (ABR threshold≤70dB, dark gray); hearing impaired mutants (left, ABR threshold 80dB and 90dB, light gray; right, ABR threshold = 80dB, light gray) and their littermate controls (black). Error bars; ±SEM. p-values; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ns = not significant; t-test.
Edn3-Ednrb signaling is essential not only in neural crest but also in placode lineage for hearing
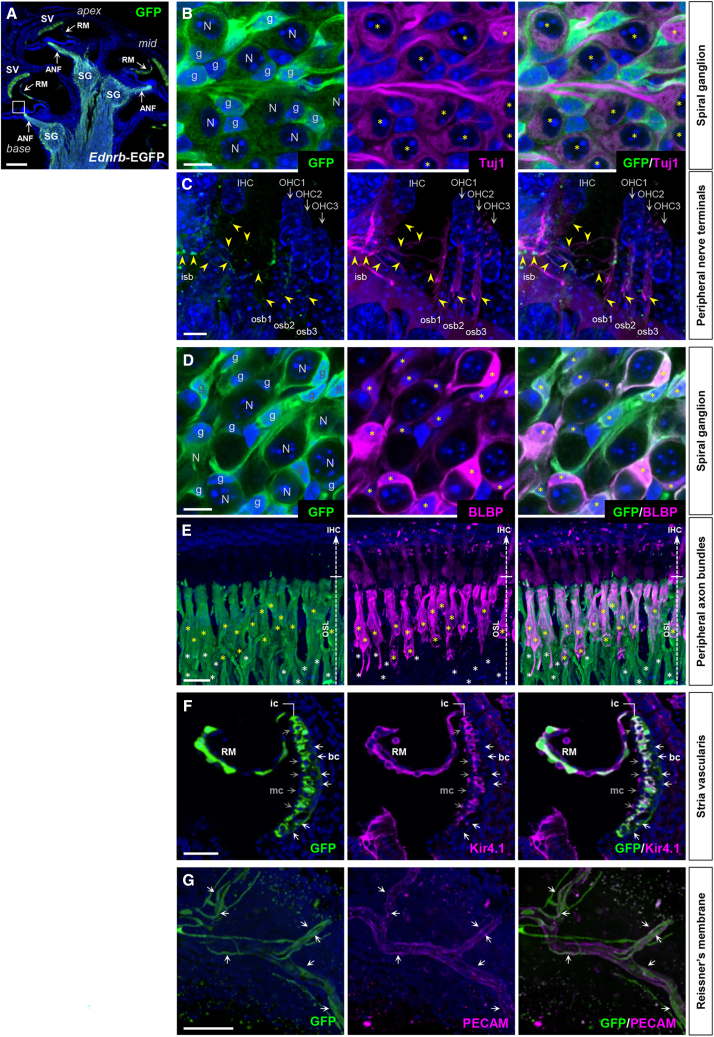
We defined the sites of Ednrb expression in the cochlea using Ednrb-eGFP BAC transgenic mice at P7, a time at which synaptic refinement is just underway shortly before the onset of hearing (P10-13).7,19 Ednrb expression was detected by GFP immunofluorescence in the lateral wall of the cochlea (stria vascularis) as well as along the entire axis of the spiral ganglia (Figure 2A). In the spiral ganglia, Ednrb expression was associated with Tuj1+ SGNs (Figure 2B) and Tuj1+ (including both afferent and efferent) nerve fibers innervating both IHCs and OHCs (Figures 2C–2E), and with BLBP+ satellite glia (Figure 2D) and BLBP+ and BLBP− Schwann cells (Figure 2E). In the stria vascularis, Kir4.1 channel-expressing intermediate cells and Kir4.1− basal cells expressed Ednrb-EGFP but not Kir4.1− marginal cells (Figure 2F). Many but not all epithelial cells of Reissner’s membrane (Figure 2F) and subsets of PECAM+ vascular endothelial cells throughout the cochlea (including ones within Reissner’s membrane; Figure 2G) also expressed Ednrb. We did not detect Ednrb expression in mechanosensory hair cells (IHCs and OHCs) or supporting cells (Figure 2C).
Figure 2.
Ednrb expression in P7 cochlea
(A–G) Sagittal sections of cochlea isolated from P7 Ednrb-EGFP BAC transgenic mice stained for GFP only (green; A) or co-stained for neuronal Tuj1 (magenta; B and C), glial BLBP (magenta; D and E), inward rectifier potassium channel Kir4.1 (magenta; F), and vascular endothelial PECAM (magenta; G), and counterstained with DAPI (blue). High magnification views demonstrate Ednrb expression in SGN cell bodies (B) and peripheral nerve terminals (C), in satellite glia in the spiral ganglia (D) and peripheral axon bundles (E), in Kir4.1+ intermediate and Kir4.1- basal stria cells (F), and in vascular endothelial cells of the Reissner’s membrane (G, flat mount view). “N” and “g” in (B) and (D) denote spiral ganglion neurons and glia, respectively. Yellow asterisks in (B) denote GFP+; Tuj1+ spiral ganglion neurons. Yellow arrowheads in (C) point to GFP+; Tuj1+ axons of spiral ganglion neurons that innervate the inner and outer hair cells corresponding to the boxed area in (A). GFP signal is much stronger in glia than in neurons. Yellow asterisks in (D) denote GFP+; BLBP+ satellite glia in the spiral ganglion. Among all Schwann cells that are associated with auditory nerve axonal bundles in (E), GFP+; BLBP+ cells and GFP+; BLBP- cells are denoted by yellow and white asterisks, respectively. White and gray arrows in (F) point to GFP+; Kir4.1- basal stria cells and GFP-; Kir4.1- marginal stria cells, respectively. Arrows in (G) denote GFP+; PECAM+ vascular endothelial cells of the Reissner’s membrane. Abbreviations: ANF, auditory nerve fibers; bc, basal cell; ic, intermediate cell; IHC, inner hair cell; isb, inner spiral bundle; mc, marginal cell; OHC, outer hair cell; osb, outer spiral bundle; OSL, osseous spiral lamina; RM, Reissner’s membrane; SG, spiral ganglion; SV, stria vascularis. Scale bars: 200μm (A), 10μm (B–D), 25μm (E), 100μm (F), 50μm (G).
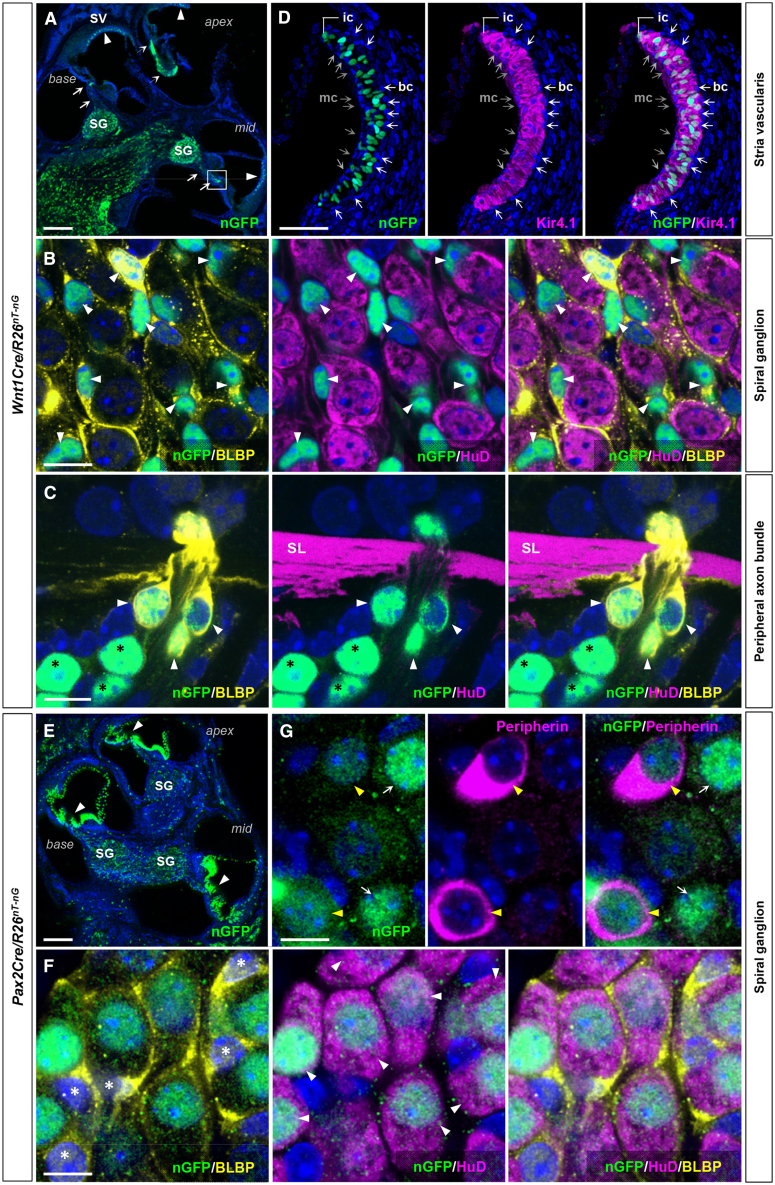
Because of this broad expression domain, we evaluated the tissue specific roles of Ednrb gene in auditory development. For this purpose, we used Wnt1Cre and Pax2Cre to drive conditional gene recombination in the neural crest and placode lineages, respectively. When combined with the conditional lineage tracer R26nT-nG which expresses nuclear Tomato before and nuclear GFP after Cre-mediated recombination, Wnt1Cre activity (visualized by nuclear GFP) was limited to BLBP+ satellite cells of the spiral ganglion (Figures 3A and 3B), BLBP+ and BLBP− Schwann cells that are associated with auditory nerve fibers (Figures 3A and 3C), and Kir4.1+ intermediate cells (melanocytes) of the stria vascularis (Figures 3A and 3D). Pax2Cre is active in SGNs (as co-labeled with the pan-neuronal marker HuD) (Figures 3E and 3F) including Peripherin+ type II SGNs (Figure 3G), and in a variety of cell types in the cochlear duct including hair cells, supporting cells, fibrocytes and epithelium (Figure 3E). There was no obvious overlap in active domains of Wnt1Cre and Pax2Cre, although because both are Cre lines they cannot be combined to confirm this point. In spiral ganglia, Pax2Cre labeled 94.8 ± 1.1% of SGNs (n = 632 from 13 cochlea) and Wnt1Cre delineated 95.4 ± 1.0% of satellite cells (n = 498 from 11 cochlea). Pax2Cre labeled only 75.63 ± 0.63% of hair cells (n = 472 from 9 cochlea) (Figure S3), although because Ednrb is not expressed in hair cells (Figure 2C), this incomplete recombination efficiency is unlikely to influence this study. Based on the immunolabeling and morphological assessment, Wnt1Cre recombination efficiency in the intermediate stria cells was 93.4 ± 1.1% (n = 151 from 10 cochlea). Additionally, we used Tbx18Cre, Phox2bCre, and Tie2Cre, which drive high efficiency and specific recombination in otic fibrocytes including basal stria cells (Figures S4A and S4B), auditory efferent nerves20 (Figures S4C and S4D), and vascular endothelial cells (Figures S4E and S4F), respectively. These tissue/cell types express Ednrb (Figures 2F and 2G) and are relevant for normal hearing.21,22,23
Figure 3.
Wnt1Cre and Pax2Cre delineate distinct cell types in the cochlea
(A–G) Sagittal sections of cochlea isolated from P7 Wnt1Cre/R26nT-nG (A–D) and Pax2Cre/R26nT-nG (E–G) mice stained for GFP (A), costained for neuronal HuD (B, C, and F), glial BLBP (B, C, and F), Kir4.1 (D), type II neuron-specific Peripherin (G), and counterstained with DAPI (blue). (A) Reporter recombination (nuclear GFP) driven by Wnt1Cre occurs in the stria vascularis (arrowheads), and in the spiral ganglion and along their peripheral nerve trajectories (arrows). (B) High magnification view of spiral ganglia in (A) shows Wnt1Cre is active in BLBP+ satellite cells (arrowheads) but not in HuD+ SGNs. (C) A magnified view of the boxed area in (A). Wnt1Cre labels BLBP+ (arrowheads) and BLBP- (black asterisks) Schwann cells (HuD-) along the peripheral nerve bundles. (D) A magnified view of the stria vascularis in (A) shows the activity of Wnt1Cre in Kir4.1+ intermediate stria cells, but not in Kir4.1- basal (white arrows) and medial (gray arrows) stria cells. (E) Reporter recombination by Pax2Cre occurs in vast majority of cochlear epithelium (arrowheads) and in the spiral ganglion. (F and G) High magnification views of spiral ganglia in (E) demonstrate that Pax2Cre is exclusively active in HuD+ SGNs (F; white arrows) including Peripherin+ type II neurons (G; yellow arrows) but not in BLBP+ satellite cells (F; asterisks). Abbreviations: SL, spiral lamina. Scale bars; 200μm (A, E), 10μm (B and C, F and G), 100μm (D).
We crossed each of these Cre drivers with the conditional Ednrb allele and performed ABR evaluation. As we recently described,18 the enteric nervous system has independent requirements for the neural crest and placode lineages such that Wnt1Cre/Ednrb and Pax2Cre/Ednrb mutants both die around P21 with HSCR. Thus, ABR analysis on conditional Ednrb mice was conducted at P19, just as done with global mutant mice described above. The analysis included 206 Wnt1Cre/Ednrb mutants (92 litters from 39 breeding pairs), 237 Pax2Cre/Ednrb mutants (103 litters from 36 breeding pairs), 12 Tbx18Cre/Ednrb mutants (4 litters from 4 breeding pairs), 15 Phox2bCre/Ednrb mutants (9 litters from 5 breeding pairs) and 16 Tie2Cre/Ednrb mutants (4 litters from 2 breeding pairs) (Figure 1A). Controls included Cre-positive and Ednrb heterozygous mice from these same litters (Table S1). Hearing impairment was evident in both Wnt1Cre/Ednrb and Pax2Cre/Ednrb with incomplete penetrance (57% and 59%, respectively), just as observed in global Ednrb and Edn3 mutant mice. Thus, Ednrb gene function is independently required in the neural crest and placode lineages for auditory function. All Tbx18Cre/Ednrb, Phox2bCre/Ednrb and Tie2Cre/Ednrb mutants exhibited ABR thresholds comparable to controls (Figures 1A and 1B), indicating a lack of Ednrb requirement in these lineages.
To further define hearing impairment observed in these mice, we tested a subset of global Ednrb (n = 8), global Edn3 (n = 14), Wnt1Cre/Ednrb (n = 15) and Pax2Cre/Ednrb (n = 13) mutants that were ABR non-responsive at 90dB for Distortion Product Otoacoustic Emissions (DPOAEs) at P20. All of these deaf mice presented a comparable DPOAE response to control mice (n = 26) in the stimulus range of 4–32 kHz, indicating that cochlear amplifier function is fully intact in all four mutant backgrounds (Figure 1C).
We further evaluated the ABR waveforms of hearing (ABR threshold ≤70dB) and hearing impaired (ABR threshold 80 or 90dB) mutants in each of these four mutant backgrounds. Wave I represents the activity of primary afferent neurons (SGNs), and its amplitude reflects the stimulus intensity and the number of mechanosensory axons that are simultaneously activated, whereas its latency reflects the duration between the initial stimulus and the peak of wave. Hearing impaired mutants of all four genotypes exhibited significantly lower wave I amplitude and longer latency compared to their controls at the stimulus intensity range (90dB and 80dB) at which they responded (Figures 1D and S1). This indicates activation of fewer SGNs and less synchronicity in SGN activation upon stimulation. Interestingly, although not as severely compromised as in hearing impaired mutants, hearing mutants (i.e., mutants with ABR threshold ≤70dB) also displayed less amplitude and longer latency relative to controls; this was statistically significant in Wnt1Cre/Ednrb and Pax2Cre/Ednrb mutants because of greater sample number but also showed the same trend in global mutants (Figure 1D). The high specificity and recombination efficiency of Pax2Cre and Wnt1Cre imply that variable recombination does not explain phenotypic range and penetrance. Rather, we suggest that additional genetic factors (phenotypic modifiers) in the diverse genetic background of our outbred colony converge with Edn3-Ednrb signaling to determine whether hearing is normal, impaired, or fully absent (see discussion).
Melanocyte deficiency is not associated with hearing impairment in Ednrb-deficient mice
Previous mouse studies with global Ednrb mutants in inbred strain backgrounds reported a complete absence of intermediate cells in the stria vascularis,14,15 and thus the etiology of hearing loss in WS4 is thought to result from impaired migration of neural crest-derived melanocytes to the stria vascularis. To address the presence or absence of cochlear melanocytes in Wnt1Cre/Ednrb mutant mice, we crossed the R26td-Tomato reporter into the Wnt1Cre/Ednrb background and visualized neural crest-derived cells at P19 in the cochlea of 6 hearing mutants and 15 hearing impaired mutants generated from 3 independent breeding pairs (Figure 4). Surprisingly, in both hearing (ABR threshold ≤70dB) and hearing impaired mutants, Wnt1Cre-labeled cells were present in the intermediate layer of the stria vascularis. Moreover, these cells were functionally mature as evidenced by expression of the inwardly rectifying potassium channel Kir4.1. In several mutants we observed various mild anomalies including a partial loss of Wnt1Cre-labeled cells (Figures 4C, 4E, and 4G), a partial loss of Kir4.1 expression in Wnt1Cre-labled intermediate stria cells (Figures 4C, 4E, and 4G), and misexpression of Kir4.1 in non-Wnt1Cre-labeled marginal cells (Figures 4C and 4G). However, these anomalies were also present in hearing mutants (Figure 4G). Significantly, the majority of hearing-impaired mutants (and of hearing mutants) had a fully normal organization of the stria vascularis. Although very few were analyzed in this manner, we also observed normal population and maturation of intermediate stria cells in global Ednrb deaf mutants (Figures 4F and 4G). Thus, in the ICR background of this colony, cochlear melanocyte defects in Ednrb mutants are variable and at most are limited in scale. More importantly, in these mice melanoblast migration or melanocyte deficiency cannot be the primary cause of hearing loss.
Figure 4.
Contribution of Wnt1Cre-labeled melanocytes to the Kir4.1+ intermediate stria cells in hearing and hearing impaired Ednrb-deficient mice
(A–G) Confocal (z stack) images of the stria vascularis of P19 hearing (ABR threshold≤70dB) Wnt1Cre/Ednrb/R26td-Tomato mutant (B and C), hearing impaired (ABR threshold = NR) Wnt1Cre/Ednrb/R26td-Tomato mutants (D and E), a littermate control (A), and hearing impaired (ABR threshold = NR) global Ednrb mutant crossed into the Wnt1Cre/R26td-Tomato background (F) immunostained for Kir4.1 (green) and counterstained with DAPI (blue). (C and E) Asterisks denote the portion of the stria vascularis that does not contain Wnt1Cre lineage derived Kir4.1+ intermediate cells. Arrows point out Wnt1Cre lineage+ cells that are lacking Kir4.1 expression. Arrowheads point out misexpression or mislocalization of Kir4.1 at the apical surface of the marginal stria cells. Scale bar: 100μm.
(G) Table summarizing the number of animals tested and the phenotype (intermediate cell number and maturation) frequency for Wnt1Cre/Ednrb and global Ednrb mutant mice.
Contribution of glial Ednrb-deficiency to defective mechanosensory synapse formation
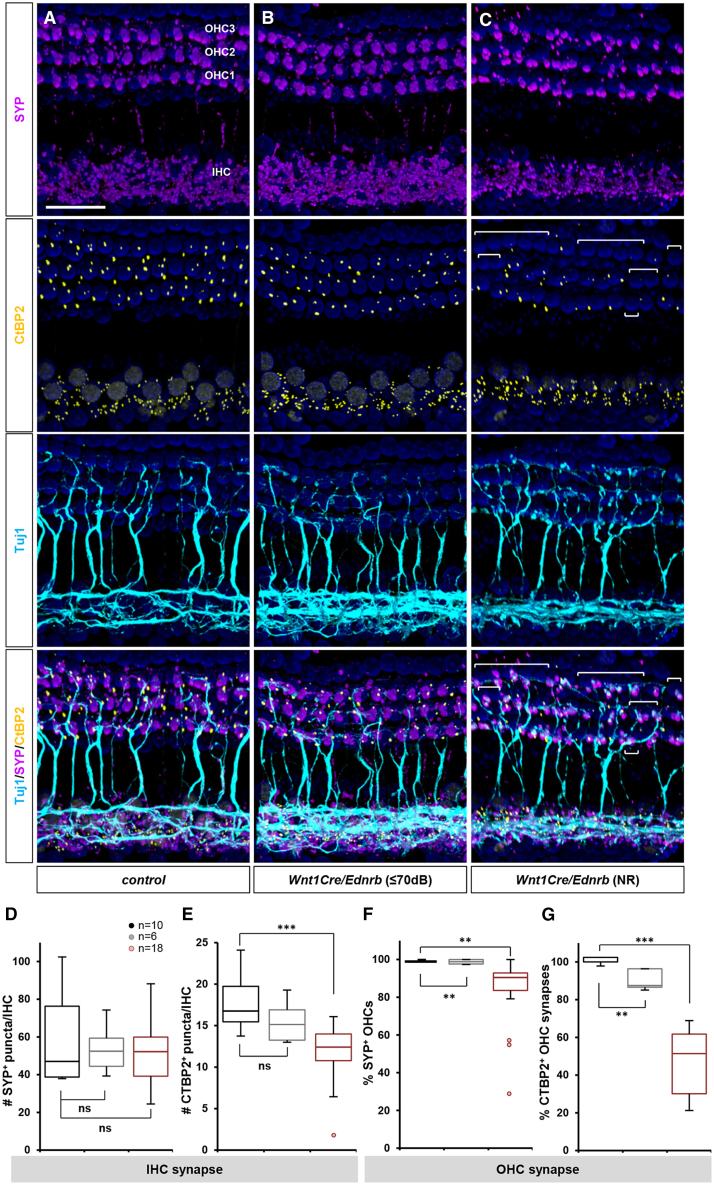
If melanocyte deficiency in the stria vascularis does not explain hearing impairment, we asked if hearing loss instead arises from auditory nervous system defects. We performed immunofluorescence analysis for Tuj1, synaptophysin (SYP), and C-terminal-binding protein 2 (CtBP2) in Wnt1Cre/Ednrb mutant cochlea to evaluate auditory nerve fibers, auditory synapses, and synaptic ribbon profiles. In wholemount (flat mount) views of control cochlea at P19, Tuj1+ nerve endings have assembled postsynaptic SYP clusters and presynaptic CtBP2 at each IHC and OHC (Figure 5A). Sagittal views confirmed that presynaptic CtBP2+ ribbons were assembled toward the basolateral surface of the IHCs and OHCs which juxtaposed with post synaptic SYP (Figures S5A–S5C). Whereas hearing mutants displayed almost normal pre- and postsynaptic assembly (Figures 5B and 5D–5G: gray), in hearing impaired mutants there was notable disorganization of SYP clusters as well as CtBP2+ ribbons on both IHCs and OHCs (Figures 5C–5G: red). This was particularly prominent at OHCs (Figure 5G), but also observed at IHCs (Figure 5E). The typical characteristic of this disorganization was poor alignment of postsynaptic SYP and presynaptic CtBP2, as shown by inconsistent size/volume of SYP clusters and more anteriorly distributed CtBP2 ribbons in both inner and OHCs (Figures S5D–S5F). Synaptic ribbons facilitate rapid, precise and continuous neurotransmission and are critical for auditory perception. Postsynaptic SYP showed a corresponding pattern, being more altered in hearing impaired OHCs (Figure 5F) than IHCs (Figure 5D). As SYP staining represents both efferent and afferent synapses, the disruption of afferent SYP+ synapses at both IHCs and OHCs in hearing impaired Wnt1Cre/Ednrb mutants is likely much greater than what is presented and quantified in Figure 5. These results imply that defective mechanosensory synapse formation is the primary phenotype that accounts for auditory impairment in Wnt1Cre/Ednrb mutants. Without proper afferent synaptic organization, auditory function would be compromised or eliminated.
Figure 5.
Defective synapse formation in hearing impaired Wnt1Cre/Ednrb mutant mice
(A–G) Whole-mount preparations of cochlea isolated from P19 Wnt1Cre/Ednrb mutant mice (B and C) and a littermate control (A) immunostained for postsynaptic SYP (magenta), presynaptic CtBP2 (yellow), neuronal Tuj1 (cyan), and counterstained with DAPI (blue). The image shown in (C) is from a hearing impaired Wnt1Cre/Ednrb mutant of median synaptic phenotype. OHCs in the bracketed areas lack CtBP2+ synaptic ribbons. Scale bar: 50μm.
(D and E) Box and whisker plots for the number of SYP+ (D) and CtBP2+ (E) puncta per IHC in control (black), hearing Wnt1Cre/Ednrb mutant (gray), and hearing impaired Wnt1Cre/Ednrb mutant (ABR threshold ≥80dB; red) mice.
(F and G) Box and whisker plots for the percentage of OHCs with postsynaptic SYP+ staining (F) and presynaptic CtBP2+ staining (G) in control (black), hearing Wnt1Cre/Ednrb mutant (gray), and hearing impaired Wnt1Cre/Ednrb mutant (ABR threshold ≥80dB; red) mice. Extremely compromised outliers in (E-F) were plotted individually and not included in the statistical analysis. p-values: ∗∗p < 0.01, ∗∗∗p < 0.001, ns = not significant; t-test.
Wnt1Cre is not active in SGNs nor in their primary afferent targets (IHCs and OHCs), all of which are placode-derived. Wnt1Cre is active in the mid/hindbrain region which includes the olivocochlear nuclei, from which efferent nerves originate. For two reasons this source is unlikely to contribute to auditory impairment in Wnt1Cre/Ednrb mutants. First, the efferent system is primarily involved in auditory discrimination and cellular protective roles rather than in primary auditory perception24,25 and so is outside the realm of ABR measurement. More decisively, Ednrb conditional mutation with efferent nerve-specific Phox2bCre did not result in hearing impairment (Figure 1A). The one remaining Wnt1Cre sublineage that could plausibly account for defective synapse formation is glia. Wnt1Cre-labeled glial cells are abundantly present in the cochlea, as satellite cells tightly associated with SGNs (Figure 3B) and as Schwann cells associated with afferent nerve fibers (Figure 3C). Satellite cells and Schwann cells both express Ednrb (Figure 2). Glial cells play important roles in many aspects of neural development and maturation, including synapse formation in both central and peripheral nervous system including auditory afferent innervation.26,27 Anatomically, there was no obvious disruption in the number or distribution of glial cells in the spiral ganglia of mutant mice (Figure S6). This suggests a functional deficiency in glial cells as the underlying condition that results in hearing impairment. Because synaptic defects observed here are between SGNs and hair cells, both of which are placode-derived, these results imply that an initial endothelin signaling process in glia, acting through the afferent axons of SGNs, remotely supports mechanosensory synapse organization at hair cells, and which is compromised in hearing impaired Wnt1Cre/Ednrb mutants.
An intrinsic requirement for Ednrb in type I SGN activation/excitation
Unlike Wnt1Cre/Ednrb mutants, hearing impaired Pax2Cre/Ednrb mutant mice displayed normal presynaptic CtBP2 and postsynaptic SYP profiles at both IHCs and OHCs (Figures S7A–S7C). This indicates that the underlying cell and molecular events that impact on auditory perception in Pax2Cre/Ednrb mutants are different from the synapse defect observed in Wnt1Cre/Ednrb mutant mice. Pax2Cre is active in the majority of otic cell types (Figure 3E), although Ednrb is not expressed in most of these that are relevant for auditory function (e.g., hair cells, supporting cells, etc.). Pax2Cre denotes subsets of Ednrb-expressing otic fibrocytes, but the normal hearing of Tbx18Cre/Ednrb mutants (Figure 1) refutes basal stria cells (Figure S4B) as the cause of hearing impairment. SGNs are the main cell type that is within the Pax2Cre recombination domain and expresses Ednrb, suggesting an intrinsic requirement for Ednrb function in SGNs. Although postsynaptic SYP labeling at sensory hair cells was normal (Figure S7), we noted a significant reduction in vesicular SYP staining in Pax2Cre/Ednrb mutant SGNs (Figure 6C). This phenotype was observed in all hearing impaired Pax2Cre/Ednrb mutants analyzed, whereas SGNs of hearing Pax2Cre/Ednrb mutants showed comparable levels of vesicular SYP+ area to control mice (Figures 6A–6D). Vesicular transport in neurons is a reflection of synaptic transmission. Thus, these observations suggest that Ednrb deficiency in SGNs results in impaired neural activation.
Figure 6.
Defective SGN activation in hearing impaired Pax2Cre/Ednrb mutant mice
(A–C) High magnification views of P19 spiral ganglion sections from hearing Pax2Cre/Ednrb (B), hearing impaired Pax2Cre/Ednrb (C) mutant mice and a littermate control (A) immunostained for SYP (green), and NF200 (magenta), and counterstained with DAPI (blue). Compiled representation of SYP+ area per SGN in each group is shown in D (see STAR Methods).
(D) Box and whisker plots for the SYP+ area per SGN in control (black), hearing Pax2Cre/Ednrb mutant (gray) and hearing impaired mutant (ABR threshold = NR; red) mice.
(E–G) Stacked confocal images of a single IHC acquired from sagittal sections of cochlea isolated from P19 hearing Pax2Cre/Ednrb (F), hearing impaired Pax2Cre/Ednrb (G) mutants and a littermate control (E) immunostained for GluR2 (yellow), and CtBP2 (magenta), and counterstained with DAPI (blue). Whole-mount views of cochlea from each given genotype immunolabeled for GluR2 and CtBP2 are shown in Figure S8. Arrowheads denote GluR2 puncta which are not juxtaposed with presynaptic CtBP2 puncta. Arrows point to CtBP2 puncta that are not juxtaposed with postsynaptic GluR2. Compiled representations of GluR2+ puncta number per IHC and GluR2/CtBP2 ratio per IHC are shown in H and I, respectively (see STAR Methods).
(H and I) Box and whisker plots for the number of GluR2+ puncta per IHC (H) and the ratio of GluR2/CtBP2 puncta (I) in control (black), hearing Pax2Cre/Ednrb mutant (gray) and hearing impaired mutant (ABR threshold = NR; red) mice. Arrows in (H) and (I) represent hearing impaired Pax2Cre/Ednrb mutants exhibiting a normal GluR2 profile at the IHC synapse that were not included in the statistical analysis. p-values: ∗∗p < 0.01, ∗∗∗p < 0.001, ns = not significant; t-test. Scale bars: 10μm (A–C), 5μm (E–G).
Glutamate is the primary neurotransmitter released by hair cells to activate SGNs, which receive this signal via AMPA receptors.28 To test if impaired glutamate signaling accounts for reduced SGN activity in hearing impaired Pax2Cre/Ednrb mutant mice, we examined the expression and localization of the AMPAR subunit GluR2 and presynaptic CtBP2. We screened a total of 17 hearing impaired Pax2Cre/Ednrb mutants and 7 hearing Pax2Cre/Ednrb mutants. In hearing Pax2Cre/Ednrb mutants, GluR2+ clusters were detected at IHCs at a comparable level to control IHCs (Figures 6F, 6H, S8B, and S8E), and each GluR2+ cluster was located in conjunction with a presynaptic CtBP2+ puncta (Figures 6F, 6I, and S8E), as also observed at IHCs of control mice (Figures 6E, 6H, 6I, S8A, and S8D). However, in hearing impaired Pax2Cre/Ednrb mutant mice, the number of GluR2+ clusters on average was significantly reduced, although those GluR2+ clusters that were present at IHCs were properly juxtaposed with CtBP2 (Figures 6G–6I, S8C, and S8F). Interestingly, this GluR2 abnormality was incompletely penetrant: it was found in 13 out of 17 hearing impaired Pax2Cre/Ednrb mutants (Figures 6H and 6I), but a subset of 4 hearing impaired mutants (Figures 6H and 6I; arrows) exhibited a normal number of GluR2+ clusters even as they still presented an impaired vesicular SYP+ profile (Figures 6C and 6D). This divergence is unlikely to simply be measurement error but more likely reflects phenotypic variation associated with the outbred background of our colony. In sum, in hearing impaired Pax2Cre/Ednrb mutants, alteration of vesicular SYP and reduced GluR2 localization together lead to the conclusion that the primary lesion is an SGN-autonomous defect in synaptic transmission. As also for Wnt1Cre/Ednrb mutants, the variable penetrance of hearing phenotypes is inferred to reflect the activity of unlinked genetic alleles (modifiers) that are variable between individual mice in this study.
Discussion
Congenital deafness is among the more prevalent chronic conditions seen in human infants. Genetic causes are thought to explain the majority of cases in developed countries. In many cases the genetic basis of deafness is unknown, and even where causative gene mutations are known the explanation for how these are transduced into auditory development or function has often been unclear. In this analysis of Edn3 and Ednrb mutant mice, we reach several new insights that have not been appreciated in prior studies. First, hearing impairment is a variable trait in endothelin signaling mutant mice as observed in human. Second, a deficiency of melanocyte migration to the stria vascularis does not explain deafness in this WS4 model. Lastly, the sites of action of endothelin signaling that account for WS4 hearing loss include not only neural crest-derived but also placode-derived cells in the cochlea, and their developmental and mechanistic roles are clearly distinct in each embryonic cell lineage. To a significant extent, these insights were possible to recognize because of the outbred diverse genetic background of our colony. In addition, we could distinguish these processes through tissue-specific conditional analysis that has not previously been conducted.
The paradigm that melanocyte migration deficiency explains deafness in Waardenburg syndromes and in particular in WS4 is strongly entrenched. This was reasonable: HSCR represents migration failure of enteric progenitors to the colon, pigmentation defects represent failure of melanocyte migration into the skin, and the intermediate cells of the stria vascularis are melanocytes that derive from neural crest and must migrate to become incorporated into the inner ear. In mouse development, melanoblasts appear in the future lateral wall of the cochlear duct as early as embryonic day 12.5 and complete migration to the intermediate layer of the stria vascularis by postnatal day 0.29,30 It is worth noting that numerous other neural crest lineages that also require migration do so normally in Edn3 and Ednrb mutants and in other WS models (e.g., dorsal root and sympathetic chain ganglia, the outflow tract of the heart, adrenal medulla, etc.), so impaired migration of stria intermediate cells in Edn3 or Ednrb mutants is not a foregone conclusion. Nonetheless, several past studies using inbred Ednrb rodent models14,15 (Edn3 has not been studied in this regard) have concluded defective migration of cochlear melanocytes as evidenced by total absence of intermediate stria cells. We do not see evidence of this in our mice. One possibility is that melanocyte migration is better supported in the strain background (ICR) of our mice. Regardless, our results indicate that endothelin signaling has multiple roles in the neural crest cell lineage of the inner ear, including in auditory synaptic assembly as observed here. This latter role has not been previously recognized.
We note that our assessment of mature melanocytes in the stria vascularis is based on the visible presence of neural crest lineage-labeled cells expressing Kir4.1, but this is not a functional assessment of endocochlear potential. It has been reported in a variety of mouse models of hearing loss that DPOAE reflects endocochlear potential31,32,33: animals with reduced DPOAEs were associated with reduced endocochlear potential, whereas animals with normal DPOAEs showed normal endocochlear potential. Thus, together with the normal presence of Kir4.1-expressing intermediate stria cells, we infer that endocochlear potentials in our hearing impaired Wnt1Cre/Ednrb mutants are normal enough to generate normal DPOAEs (Figure 1C). We have not yet ruled out if Ednrb-deficient intermediate cells influence the adjacent marginal cell functions in homeostatic regulation of endolymph Ca2+ and HCO3− (and other cations/anions) that could also influence hearing threshold.32,34,35
Alternatively, our studies implicate glial cells as a candidate site of Ednrb activity to explain deafness in Wnt1Cre/Ednrb mutants. To a certain extent, our interest in glia is based on exclusion of other candidate neural crest cell types (melanocytes and cranial efferent nerves). Formal proof of a glial-specific Ednrb role will require an appropriate glia-specific Cre line which we do not yet have. Nonetheless, such a role is quite reasonable: for example, in mice with conditional deletion of Sox10 using Wnt1Cre, satellite/Schwann cells were absent in the spiral ganglia by E16.5, and in their absence, placode-derived SGNs failed to migrate to appropriate sites and also failed to project their afferent axons to appropriate targets.27,36 Apparently, glial cells play crucial roles in guiding cochlear neuron migration and axonal growth during embryonic development at least by physical contact (and possibly by molecular interactions). In our Wnt1Cre/Ednrb mutants, along with melanocyte migration, cochlear glia migrate normally and form spiral ganglia with placode-derived SGNs at the appropriate location with no obvious defects in their size and morphology. Furthermore, Ednrb-deficient satellite cells normally express Kir4.1 and are capable of enfolding individual SGN (Figure S6C). We propose that glial endothelin signaling is dispensable in the embryonic phase of cochlear development but crucial for functional maturation of auditory circuitry: in response to Edn3 and mediated through Ednrb, glial cells remotely promote afferent synapse formation either by secreting paracrine factors that act on SGNs or through cell contact-mediated activation/suppression of relevant signaling pathways in SGNs. Additional high-resolution analysis such as transmission electron microscopy may be warranted to formally validate defective synapse formation in hearing impaired Wnt1Cre/Ednrb mutants. As presented by the variable severity of deafness in hearing impaired Wnt1Cre/Ednrb mutants (Figures 1A, 1B, and 1D), we predict that primary Ednrb-deficiency makes spiral ganglion glia vulnerable to additional and/or epistatic effects of other (modifier) genes, and which ultimately manifest impairment in synapse formation. For a better understanding of the mechanistic basis of glial Ednrb-mediated hearing loss, it will be informative to identify those modifier genes and to define how these genes independently interact with primary Ednrb-deficiency in spiral ganglion glia.
Our studies have also revealed a placode-specific role for endothelin signaling in auditory functionality. A hint of this role appeared in one prior study in which transgenic re-expression of Ednrb in SGNs of global Ednrb mutant mice resulted in partial improvement in hearing.14 This was associated with rescue of SGN neurodegeneration. We did not observe SGN neurodegeneration in our global and conditional mutant mice, which is possibly because neural survival is better supported in the ICR background or because the postnatal day 19 time point that we use for our study (due to HSCR lethality) is not old enough to detect neurodegeneration. We also did not observe any alteration in spiral ganglion size and morphology including number and distribution of Peripherin+ type II neurons as previously described.37 Instead, our data implicate SGN synaptic transmission as the primary lesion in Pax2Cre/Ednrb mutants. The one common feature of all hearing impaired Pax2Cre/Ednrb mutants was a reduction of SYP+ vesicles, which is an indication of diminished synaptic activity. We observed diminished synaptic GluR2 in most but not all deaf mutants; this is a likely contributor to diminished vesicular transport in at least these mice. One possibility is that Ednrb regulates GluR2 expression or localization, although if so there are clearly additional mechanisms involved that account for normal GluR2 in hearing Pax2Cre/Ednrb mutants and as well in a small subset of deaf mutants.
We believe that genetic modifiers in the outbred ICR strain background of our colony account for the variable penetrance of hearing impairment in global and conditional mutant backgrounds. Strain background can also explain variability in GluR2 localization in Pax2Cre/Ednrb mutants and perhaps also our observation of normal melanocyte presence in the stria vascularis compared to past reports.
In most cases, a controlled inbred strain background is a benefit in terms of maintaining phenotypic consistency. However, as described here, the phenotypic uniformity of past studies is likely to have obscured other important features of auditory maturation that are under Ednrb control. We were able to recognize these new features in the outbred background of our colony by examining a very large number of mice for all phenotypes and by segregating them through lineage-specific conditional mutagenesis. It is obvious that EDN3 and EDNRB mutations in humans have variable phenotypic presentation,8 which is a clear indication of the impact of modifier genes. Our colony resembles the human situation more accurately than any inbred background can replicate. New insights on the cell and molecular biology of auditory development that we are able to achieve with our colony may lead to new diagnostic criteria and perhaps also new therapeutic opportunities.
Limitations of the study
While we demonstrate divergent utilization of endothelin signaling in neural crest-derived glia and placode-derived auditory sensory neurons to establish functional auditory synapses, immunofluorescence localization of pre- and postsynaptic components does not establish the full nature of the synaptic defects. Additional studies, such as electrophysiological recordings, would provide a better definition of the observed presynaptic and postsynaptic defects. As noted in the text, our assignment of Ednrb function within the neural crest lineage to glia is primarily based on exclusion of all other candidate cell types, and confirmation with a glial-specific Cre line will be valuable. Finally, our observations do not reconcile why different explanations for auditory dysfunction were reached in previous studies. We suggest that strain background is a major feature and, in this light, it would be worthwhile to examine auditory function, stria vascularis melanocyte presence, and cochlear synaptic organization at P19 (as in this study) in mutant mice on different backgrounds. Similarly, if the auditory defects seen in this study on the ICR background can be uncoupled from HSCR lethality (for example, by suitable genetic manipulation), it will be particularly informative to determine if melanocyte presence in the stria vascularis changes in mice that reach the older ages examined in prior studies.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Takako Makita (makita@musc.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
ABR and microscopy data reported in this paper will be shared by the lead contact upon request.
-
•
This study did not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
No acknowledgments to provide.
Author contributions
T.M. and H.M.S. conceptualized the project and its design, J.T., A.D., and T.M. performed experiments and collected data. All authors participated in writing and editing drafts of the manuscript.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit Polyclonal BLBP (Fabp7) antibody | Abcam | Cat# ab32423; RRID:AB_880078 |
| Mouse Anti-CtBP2 (Ctbp2) Monoclonal Antibody, Unconjugated, Clone 16 | BD Biosciences | Cat# 612044; RRID:AB_399431 |
| Chicken Polyclonal GFP antibody | Abcam | Cat# ab13970; RRID:AB_300798 |
| Anti-Glutamate Receptor 2 (GluR2/Gria2), extracellular, clone 6C4 | Millipore | Cat# MAB397; RRID:AB_2113875 |
| Mouse monoclonal anti-HuD (Elavl4) antibody (E-1) | Santa Cruz Biotechnology | Cat# sc-28299; RRID:AB_627765 |
| Guinea pig Anti-Kir4.1 (KCNJ10) Antibody | Alomone Labs | Cat# APC-035-GP; RRID:AB_2340962 |
| Rat anti-CD31 (PECAM1) Antibody | BD Biosciences | Cat# 550274; RRID:AB_393571 |
| Rabbit Recombinant Monoclonal Peripherin (Prph) antibody | Abcam | Cat# ab99942; RRID:AB_10863617 |
| Rabbit Anti-Synaptophysin (SYP) Polyclonal Antibody, Unconjugated | Abcam | Cat# ab32594; RRID:AB_778204 |
| Mouse monoclonal anti-Synaptophysin (SYP) Antibody (D-4) | Santa Cruz Biotechnology | Cat# sc-17750; RRID:AB_628311 |
| Neuronal Class III beta-Tubulin (TUJ1) Monoclonal Antibody, Purified | BioLegend | Cat# MMS-435P; RRID:AB_2313773 |
| Goat anti-Chicken IgY (H+L) Secondary Antibody, Alexa Fluor™ 488 | Thermo Fisher Scientific | Cat# A-11039; RRID:AB_2534096 |
| Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 | Thermo Fisher Scientific | Cat# A-11001 (also A11001, A 11001); RRID:AB_2534069 |
| Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 | Thermo Fisher Scientific | Cat# A-11008 (also A11008); RRID:AB_143165 |
| Rabbit anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 594 | Thermo Fisher Scientific | Cat# A-11062; RRID:AB_2534109 |
| Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 594 | Thermo Fisher Scientific | Cat# A-11012; RRID:AB_2534079 |
| Rabbit anti-Rat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 594 | Thermo Fisher Scientific | Cat# A-21211; RRID:AB_2535797 |
| Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 647 | Thermo Fisher Scientific | Cat# A-21235; RRID:AB_2535804 |
| Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 647 | Thermo Fisher Scientific | Cat# A-21244; RRID:AB_2535812 |
| Goat anti-Guinea Pig IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 647 | Thermo Fisher Scientific | Cat# A-21450; RRID:AB_2535867 |
| Chemicals, peptides, and recombinant proteins | ||
| Paraformaldehyde | VWR | Cat# IC150146.5; CAS: 30525-89-4 |
| EDTA | VWR | Cat# BDH9232; CAS: 60-00-4 |
| D-(+)-Sucrose | VWR | Cat# 97061-428; CAS: 57-50-1 |
| TRITON™ X-100 | VWR | Cat# EM-9410; CAS: 9002-93-1 |
| Urea | VWR | Cat# BDH4602; CAS: 57-13-6 |
| Glycerol | Fisher Scientific | Cat# 60-048-020; CAS: 56-81-5 |
| DAPI (4',6-Diamidino-2-Phenylindole, Dihydrochloride) | Thermo Fisher Scientific | Cat# D1306; CAS: 28718-91-4 |
| Experimental models: Organisms/strains | ||
| Mouse: B6;129-Ednrbtm1.1Nat/J | Rattner et al.38 | RRID:IMSR_JAX:011080 |
| Mouse: 129-Edn3tm1Ywa/J | Baymash et al.12 | RRID:IMSR_JAX:002516 |
| Mouse: STOCK H2az2Tg(Wnt1-cre)11Rth Tg(Wnt1-GAL4)11Rth/J | Daniellian et al.39 | RRID:IMSR_JAX:003829 |
| Mouse: Tg(Pax2-cre)1Akg | Ohyama et al.40 | RRID:MGI:4438962 |
| Mouse: B6(Cg)-Tg(Phox2b-cre)3Jke/J | Scott et al.41 | RRID:IMSR_JAX:016223 |
| Mouse: Tbx18tm2.1(cre)Sev | Cai et al.42 | N/A |
| Mouse: B6.Cg-Tg(Tek-cre)1Ywa/J | Kinusaki et al.43 | RRID:IMSR_JAX:008863 |
| Mouse: B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | The Jackson Laboratory | RRID:IMSR_JAX:007914 |
| Mouse: B6N.129S6-Gt(ROSA)26Sortm1(CAG-tdTomato∗.-EGFP∗)Ees/J | The Jackson Laboratory | RRID:IMSR_JAX:023537 |
| Mouse: Tg(Ednrb-EGFP)EP59Gsat/Mmucd | MMRRC | RRID:MGI:3842964 |
| Software and algorithms | ||
| BioSigRZ | Tucker-Davis Technologies | RRID:SCR_014820, http://www.tdt.com/biosigrz.html |
| Fiji | NIH | RRID:SCR_002285, http://fiji.sc |
| FociPicker3D | Du et al.47 | https://imagej.net/ij/plugins/foci-picker3d/index.html |
Experimental model and study participant details
Animals
Ednrb38 (JAX:011080), Edn312 (JAX:002516), Wnt1Cre39 (JAX:003829), Pax2Cre40 (MMRRC:010569-UNC), Phox2bCre41 (JAX: 016223), Tbx18Cre,42 Tie2Cre43 (JAX:008863), ROSA26CAG-tdTomato44 (JAX: 007914), ROSA26nT-nG45 (JAX: 023537), Ednrb-EGFP46 (MMRRC_010620-UCD) alleles have been described previously. All experiments with animals complied with National Institutes of Health guidelines and were reviewed and approved by the Medical University of South Carolina Institutional Animal Care and Use Committee. Wild-type ICR mice used for propagation of these lines were obtained from Harlan/Envigo. All lines used in this study have been backcrossed for numerous generations to the ICR background, although because of intercrosses this was not rigorously controlled or quantified.
Method details
Auditory brain response (ABR) and distortion product of otoacoustic emissions (DPOAE)
Postnatal day 19 mice were anesthetized with 3.0% isoflurane inhalation via a SomnoSuite small animal anesthesia system (Kent Scientific), and placed on a heating pad inside the SD1 small test enclosure (EST-LINDGREN). Needle electrodes were placed subcutaneously at the vertex (active), the ipsilateral ear (reference) and the pelvic limb (ground), and click-evoked ABR was recorded by the RZ6 signal processor (TDT) in declining 10dB steps beginning at 90dB. The ABR threshold was determined as the lowest intensity at which a recognizable wave I ABR waveform could be identified. The MF1 multi-field magnetic speaker (calibrated with BioSigRZ software (TDT) prior to each use) was used for open field ABR recording. BioSigRZ was used for ABR waveform (wave I amplitude and latency) analysis. DPOAE measurements were performed at P20 on a subset of controls and mutant mice that were found to be nonresponsive (NR) at P19. The acoustic probe containing the MF1 multi-field magnetic speakers and the EB10+ DPOAE microphone (TDT) was calibrated for closed field experiments and placed in the ear canal. DPOAE data were collected in response to a combination of two pure tones presented continuously at the primary frequency f1 and f2, and measured at the audiometric frequencies of 4kHz, 8kHz, 16kHz and 32kHz by the RZ6 signal processor (TDT). The primary frequency of f1 is the audiometric frequency multiplied by a factor of 0.909, and the frequency of f2 is the audiometric frequency multiplied by a factor of 1.09, and thus the frequency ratio f2/f1=1.2. Acoustic responses and DPOAE amplitudes (fDP=2f1-f2) with the noise floor (signal to noise ratio) were analyzed using BioSigRZ Software (TDT).
Cochlea preparation for histology and immunostaining
Postnatal day 20 mice were anesthetized and transcardially perfused with PBS followed by 4% paraformaldehyde. The inner ears were dissected, pierced at the apical wall of the cochlea, and postfixed in 4% PFA for 2 hr at room temperature. The entire inner ear was washed with PBS, decalcified in 8% EDTA (pH7.4) for 2-3 days, and then washed in PBS. The whole tissue was embedded in 8% low melt agarose, then vibratome sectioned at 100μm thickness.
Immunofluorescence staining and imaging
Inner ear sections and wholemount cochlear preparations were cryoprotected with 30% sucrose/PBS, permeabilized by three freeze-thaw cycles, and washed extensively with PBS to remove sucrose. Tissues were incubated with primary antibodies at 37°C overnight, and then with Alexa Fluor secondary antibodies (Invitrogen) for 3-5 hours with gentle agitation. Primary antibodies used in this study include BLBP (1:500; Abcam ab32423) , CtBP2 (1:500; BD 612044), GFP (1:3000; Abcam ab13970), GluR2 (1:300; Millipore MAB397), HuD (1:500; SCBT sc-28299), Kir4.1 (1:400; Alomone AGP-035-GP), PECAM1/CD31 (1:200; BD 550274), Peripherin (1:200; Abcam ab99942), SYP (1:500, Abcam ab32594, 1:500, SCBT sc-17750), Tuj1 (1:1000; BioLegend MMS-435P). All immunostained sections/tissues were counterstained with DAPI, and then cleared in ScaleU2 (4M urea, 30% glycerol, 0.1% Triton-X 100). Fluorescence images were acquired using a Leica SPE confocal microscope system. To quantify the recombination efficiency (Figures 3 and S3), five confocal images (15μm apart) were extracted from z-stack images (1μm interval) from one sagittal section containing all three turns from each cochlea analyzed, and Rosa reporter positive cells in HuD+ SGN neurons, BLBP+ satellite glia, hair cells (by anatomical location), and Kir4.1+ intermediate stria cells in the base through midturn were counted using the Fiji ImageJ plugin Cell Counter. To quantify IHC and OHC synaptic components (Figure 5), three confocal z-stack images (0.5μm intervals) were acquired from base to middle turn of each cochlea and the number/volume of each fluorescence puncta was measured using the Fiji ImageJ plugin FociPicker3D.47 The average of three measurements per cochlea was used for compiling data. To quantify vesicular synaptophysin in SGNs (Figures 6A–6C), five confocal images (15μm apart) were extracted from z-stack images (1μm interval) from each tissue section, and SYP+ area on each was measured by binary threshold selection using ImageJ normalized to the number of SGNs per image. The average of the five measurements per tissue was used for compiling data. To quantify IHC excitatory synapse components (Figures 6D–6F), five confocal z-stack images (0.5μm intervals) were acquired from base to middle turn of each cochlea and the number/volume of each fluorescence puncta was measured using the Fiji ImageJ plugin FociPicker3D. The average of five measurements per cochlea was used for compiling data.
Quantification and statistical analysis
All quantified data were graphed as mean±SEM, and analyzed for significance using a two-tailed Student’s t-test. In all figures, significance is denoted with a single asterisk for p<0.05, a double asterisk for p<0.01, and a triple asterisk for p<0.001. Box and whisker plots show the mean, first and third quartiles, and full range of data except where explicitly indicated. Individually plotted outliers (Figures 5E, 5F, 6H, and 6I) were not included in the box and whisker plots.
Published: December 24, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111680.
Supplemental information
References
- 1.Barald K.F., Kelley M.W. From placode to polarization: new tunes in inner ear development. Development. 2004;131:4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- 2.Ladher R.K., O'Neill P., Begbie J. From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development. 2010;137:1777–1785. doi: 10.1242/dev.040055. [DOI] [PubMed] [Google Scholar]
- 3.Zine A., Fritzsch B. Early Steps towards Hearing: Placodes and Sensory Development. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms24086994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyott S.J., Pavlinkova G., Yamoah E.N., Fritzsch B. Harmony in the Molecular Orchestra of Hearing: Developmental Mechanisms from the Ear to the Brain. Annu. Rev. Neurosci. 2024;47:1–20. doi: 10.1146/annurev-neuro-081423-093942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steel K.P., Barkway C. Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development. 1989;107:453–463. doi: 10.1242/dev.107.3.453. [DOI] [PubMed] [Google Scholar]
- 6.D'Amico-Martel A., Noden D.M. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am. J. Anat. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- 7.Johnson S.L., Safieddine S., Mustapha M., Marcotti W. Hair Cell Afferent Synapses: Function and Dysfunction. Cold Spring Harb. Perspect. Med. 2019;9 doi: 10.1101/cshperspect.a033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pingault V., Ente D., Dastot-Le Moal F., Goossens M., Marlin S., Bondurand N. Review and update of mutations causing Waardenburg syndrome. Hum. Mutat. 2010;31:391–406. doi: 10.1002/humu.21211. [DOI] [PubMed] [Google Scholar]
- 9.Gettelfinger J.D., Dahl J.P. Syndromic Hearing Loss: A Brief Review of Common Presentations and Genetics. J. Pediatr. Genet. 2018;7:1–8. doi: 10.1055/s-0037-1617454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Read A.P., Newton V.E. Waardenburg syndrome. J. Med. Genet. 1997;34:656–665. doi: 10.1136/jmg.34.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S., Song J., He C., Cai X., Yuan K., Mei L., Feng Y. Genetic insights, disease mechanisms, and biological therapeutics for Waardenburg syndrome. Gene Ther. 2022;29:479–497. doi: 10.1038/s41434-021-00240-2. [DOI] [PubMed] [Google Scholar]
- 12.Baynash A.G., Hosoda K., Giaid A., Richardson J.A., Emoto N., Hammer R.E., Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 13.Hosoda K., Hammer R.E., Richardson J.A., Baynash A.G., Cheung J.C., Giaid A., Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 14.Ida-Eto M., Ohgami N., Iida M., Yajima I., Kumasaka M.Y., Takaiwa K., Kimitsuki T., Sone M., Nakashima T., Tsuzuki T., et al. Partial requirement of endothelin receptor B in spiral ganglion neurons for postnatal development of hearing. J. Biol. Chem. 2011;286:29621–29626. doi: 10.1074/jbc.M111.236802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushima Y., Shinkai Y., Kobayashi Y., Sakamoto M., Kunieda T., Tachibana M. A mouse model of Waardenburg syndrome type 4 with a new spontaneous mutation of the endothelin-B receptor gene. Mamm. Genome. 2002;13:30–35. doi: 10.1007/s00335-001-3038-2. [DOI] [PubMed] [Google Scholar]
- 16.Deol M.S. The neural crest and the acoustic ganglion. J. Embryol. Exp. Morphol. 1967;17:533–541. [PubMed] [Google Scholar]
- 17.Dang R., Torigoe D., Suzuki S., Kikkawa Y., Moritoh K., Sasaki N., Agui T. Genetic background strongly modifies the severity of symptoms of Hirschsprung disease, but not hearing loss in rats carrying Ednrb(sl) mutations. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poltavski D.M., Cunha A.T., Tan J., Sucov H.M., Makita T. Lineage-specific intersection of endothelin and GDNF signaling in enteric nervous system development. Elife. 2024;13 doi: 10.7554/eLife.96424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulankina A.V., Moser T. Neural circuit development in the mammalian cochlea. Physiology. 2012;27:100–112. doi: 10.1152/physiol.00036.2011. [DOI] [PubMed] [Google Scholar]
- 20.Macova I., Pysanenko K., Chumak T., Dvorakova M., Bohuslavova R., Syka J., Fritzsch B., Pavlinkova G. Neurod1 Is Essential for the Primary Tonotopic Organization and Related Auditory Information Processing in the Midbrain. J. Neurosci. 2019;39:984–1004. doi: 10.1523/JNEUROSCI.2557-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Bonito M., Bourien J., Tizzano M., Harrus A.G., Puel J.L., Avallone B., Nouvian R., Studer M. Abnormal outer hair cell efferent innervation in Hoxb1-dependent sensorineural hearing loss. PLoS Genet. 2023;19 doi: 10.1371/journal.pgen.1010933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furness D.N. Forgotten Fibrocytes: A Neglected, Supporting Cell Type of the Cochlea With the Potential to be an Alternative Therapeutic Target in Hearing Loss. Front. Cell. Neurosci. 2019;13:532. doi: 10.3389/fncel.2019.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trune D.R., Nguyen-Huynh A. Vascular Pathophysiology in Hearing Disorders. Semin. Hear. 2012;33:242–250. doi: 10.1055/s-0032-1315723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashmore J.F., Oghalai J.S., Dewey J.B., Olson E.S., Strimbu C.E., Wang Y., Shera C.A., Altoè A., Abdala C., Elgoyhen A.B., et al. The Remarkable Outer Hair Cell: Proceedings of a Symposium in Honour of W. E. Brownell. J. Assoc. Res. Otolaryngol. 2023;24:117–127. doi: 10.1007/s10162-022-00852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elgoyhen A.B., Katz E. The efferent medial olivocochlear-hair cell synapse. J. Physiol. Paris. 2012;106:47–56. doi: 10.1016/j.jphysparis.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanani M., Verkhratsky A. Satellite Glial Cells and Astrocytes, a Comparative Review. Neurochem. Res. 2021;46:2525–2537. doi: 10.1007/s11064-021-03255-8. [DOI] [PubMed] [Google Scholar]
- 27.Mao Y., Reiprich S., Wegner M., Fritzsch B. Targeted deletion of Sox10 by Wnt1-cre defects neuronal migration and projection in the mouse inner ear. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reijntjes D.O.J., Pyott S.J. The afferent signaling complex: Regulation of type I spiral ganglion neuron responses in the auditory periphery. Hear. Res. 2016;336:1–16. doi: 10.1016/j.heares.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Bonnamour G., Charrier B., Sallis S., Leduc E., Pilon N. NR2F1 regulates a Schwann cell precursor-vs-melanocyte cell fate switch in a mouse model of Waardenburg syndrome type IV. Pigment Cell Melanoma Res. 2022;35:506–516. doi: 10.1111/pcmr.13054. [DOI] [PubMed] [Google Scholar]
- 30.Renauld J.M., Khan V., Basch M.L. Intermediate Cells of Dual Embryonic Origin Follow a Basal to Apical Gradient of Ingression Into the Lateral Wall of the Cochlea. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.867153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingham N.J., Banafshe N., Panganiban C., Crunden J.L., Chen J., Lewis M.A., Steel K.P. Inner hair cell dysfunction in Klhl18 mutant mice leads to low frequency progressive hearing loss. PLoS One. 2021;16 doi: 10.1371/journal.pone.0258158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian C., Johnson K.R., Lett J.M., Voss R., Salt A.N., Hartsock J.J., Steyger P.S., Ohlemiller K.K. CACHD1-deficient mice exhibit hearing and balance deficits associated with a disruption of calcium homeostasis in the inner ear. Hear. Res. 2021;409 doi: 10.1016/j.heares.2021.108327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia A., Visosky A.M.B., Cho J.H., Tsai M.J., Pereira F.A., Oghalai J.S. Altered traveling wave propagation and reduced endocochlear potential associated with cochlear dysplasia in the BETA2/NeuroD1 null mouse. J. Assoc. Res. Otolaryngol. 2007;8:447–463. doi: 10.1007/s10162-007-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wangemann P., Itza E.M., Albrecht B., Wu T., Jabba S.V., Maganti R.J., Lee J.H., Everett L.A., Wall S.M., Royaux I.E., et al. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med. 2004;2:30. doi: 10.1186/1741-7015-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wangemann P., Nakaya K., Wu T., Maganti R.J., Itza E.M., Sanneman J.D., Harbidge D.G., Billings S., Marcus D.C. Loss of cochlear HCO3- secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am. J. Physiol. Renal Physiol. 2007;292:F1345–F1353. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szeto I.Y.Y., Chu D.K.H., Chen P., Chu K.C., Au T.Y.K., Leung K.K.H., Huang Y.H., Wynn S.L., Mak A.C.Y., Chan Y.S., et al. SOX9 and SOX10 control fluid homeostasis in the inner ear for hearing through independent and cooperative mechanisms. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2122121119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott K.L., Kersigo J., Lee J.H., Jahan I., Pavlinkova G., Fritzsch B., Yamoah E.N. Developmental Changes in Peripherin-eGFP Expression in Spiral Ganglion Neurons. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.678113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rattner A., Yu H., Williams J., Smallwood P.M., Nathans J. Endothelin-2 signaling in the neural retina promotes the endothelial tip cell state and inhibits angiogenesis. Proc. Natl. Acad. Sci. USA. 2013;110:E3830–E3839. doi: 10.1073/pnas.1315509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danielian P.S., Muccino D., Rowitch D.H., Michael S.K., McMahon A.P. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 40.Ohyama T., Groves A.K. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- 41.Scott M.M., Williams K.W., Rossi J., Lee C.E., Elmquist J.K. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J. Clin. Invest. 2011;121:2413–2421. doi: 10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai C.L., Martin J.C., Sun Y., Cui L., Wang L., Ouyang K., Yang L., Bu L., Liang X., Zhang X., et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kisanuki Y.Y., Hammer R.E., Miyazaki J., Williams S.C., Richardson J.A., Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 44.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prigge J.R., Wiley J.A., Talago E.A., Young E.M., Johns L.L., Kundert J.A., Sonsteng K.M., Halford W.P., Capecchi M.R., Schmidt E.E. Nuclear double-fluorescent reporter for in vivo and ex vivo analyses of biological transitions in mouse nuclei. Mamm. Genome. 2013;24:389–399. doi: 10.1007/s00335-013-9469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong S., Doughty M., Harbaugh C.R., Cummins A., Hatten M.E., Heintz N., Gerfen C.R. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du G., Drexler G.A., Friedland W., Greubel C., Hable V., Krücken R., Kugler A., Tonelli L., Friedl A.A., Dollinger G. Spatial dynamics of DNA damage response protein foci along the ion trajectory of high-LET particles. Radiat. Res. 2011;176:706–715. doi: 10.1667/rr2592.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
ABR and microscopy data reported in this paper will be shared by the lead contact upon request.
-
•
This study did not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.